Neuronal c-Abl overexpression leads to neuronal loss and neuroinflammation in the mouse forebrain - PubMed (original) (raw)
Comparative Study
Neuronal c-Abl overexpression leads to neuronal loss and neuroinflammation in the mouse forebrain
Sarah D Schlatterer et al. J Alzheimers Dis. 2011.
Abstract
Several immunocytochemical studies have revealed that Abelson tyrosine kinase (c-Abl) is associated with both neuritic plaques and neurofibrillary tangles in the brains of patients with Alzheimer's disease (AD). Additionally, c-Abl has been shown to phosphorylate tau on tyrosine 394. The activity of c-Abl is also involved in the control of the cell cycle and apoptosis. To examine the consequences of c-Abl activation in the adult brain, we have constructed two lines of transgenic mice expressing either a constitutively active form of c-Abl (AblPP/tTA mice) or its sister protein, Arg (ArgPP/tTA mice), with a neuron-specific promoter (CamKIIα) regulated by doxycycline (Tet-Off). Expression of active c-Abl in adult mouse forebrain neurons results in severe neurodegeneration, particularly in the CA1 region of the hippocampus. Neuronal loss was preceded and accompanied by substantial microgliosis and astrocyctosis. Despite careful examination, no c-Abl expression is found in glial cells, indicating that neuronal c-Abl expression is responsible for the gliosis. In contrast, ArgPP/tTA mice have no evidence of neuronal loss or gliosis, even though protein expression and kinase activity levels are similar to those in the AblPP/tTA mice. Given the evidence of c-Abl activation in the human AD brain combined with the pathological phenotype of AblPP/tTA mice, it is likely that aberrant c-Abl activity may play a role in neurodegenerative disease.
Figures
Fig. 1
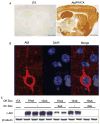
Abl expression in AblPP/tTA mice. A) c-Abl is expressed and active in AblPP/tTA mice. Representative sections of single transgenic control mouse and AblPP/tTA mouse 3 weeks off dox stained for c-Abl (K12). B) c-Abl (K12) is present in cell bodies of neurons in AblPP/tTA mice. Immunofluorescence (K12 – red, DAPI – blue) of cortex of an AblPP/tTA mouse 2 weeks off dox. C) Abl expression is doxycyline dependent. Abl (2411) immunoblot of AblPP/tTA mouse cortex 10 weeks off dox with or without introduction of dox back into diet for 2 or 4 weeks.
Fig. 2
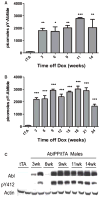
Abl activity in AblPP/tTA mice. A) Male AblPP/tTA mice. B) Female AblPP/tTA mice. Enzyme activity ELISAs of AblPP/tTA mouse cortex. Average picomoles of Abltide phosphorylated per kinase reaction, n = 4 per timepoint. One-way ANOVA with Dunnett’s post-test was used to calculate significance of Abltide phosphorylation in AblPP/tTA mice vs. single transgenic (tTA) controls. *p < 0.05, **p < 0.01, ***p< 0.001. C) Total c-Abl (2411) and phospho-Abl (pY412) immunoblots of male AblPP/tTA mouse cortex 3, 6, 9, 11, and 14 weeks off dox.
Fig. 3
Microgliosis in AblPP/tTA mice. Iba1 immunohistochemistry of AblPP/tTA male and female hippocampi. Top row shows 4 separate single transgenic control mice. Subsequent rows show 4 different mice per timepoint, with #wk indicating weeks off dox. Scale bars = 2 mm.
Fig. 4
Reactive Astrocytosis in AblPP/tTA mice. GFAP immunohistochemistry of AblPP/tTA males and females throughout the hippocampus. Top row shows 4 separate single transgenic control mice. Subsequent rows show 4 different mice per timepoint, with #wk indicating weeks off dox. Scale bars = 2 mm.
Fig. 5
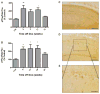
Neurodegeneration in CA1 of AblPP/tTA mice. A) NeuN staining of AblPP/tTA male and female mice CA1. Top row shows 4 separate single transgenic control mice. Subsequent rows show 4 different mice per timepoint, with #wk indicating weeks off dox. Scale bars = 800 μm. B) NeuN immunohistochemistry of control vs. male and female AblPP/tTA mouse hippocampi. Scale bars = 2 mm.
Fig. 6
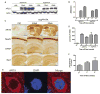
Tau hyperphosphorylation in AblPP/tTA mice. A, B) ELISA analysis of tyrosine phosphorylated (4G10) tau/total (DA9) tau in male (A) and female (B) AblPP/tTA mice. n = 4 for each timepoint. One-way ANOVA with Dunnett’s post-test was used to calculate whether AblPP/tTA mice showed significant increases in tau phosphorylation when compared to control (tTA) mice. *p < 0.05, **p < 0.01, ***p < 0.001. C–E) PHF1 staining of representative sections of AblPP/tTA mouse CA1 (D, E) versus controls (C). Scale bars = 800 μm (C, D), 400 μm (E).
Fig. 7
ArgPP/tTA mice do not differ significantly from controls. A) AblPP/tTA and ArgPP/tTA exhibit similar levels of protein expression. Western blotting with AR23 antibody of AblPP/tTA and ArgPP/tTA mouse cortex homogenates 12 and 24 weeks off doxycycline. B) Densitometry analysis of Western blotting in 7A. No significant differences were found between AblPP and ArgPP expression in the two lines of mice. C) Representative sections of control mice and ArgPP/tTA mice 24 and 30 weeks off dox stained for AR19, NeuN, Iba1, and GFAP. Scale bars = 8 mm (AR19), 800 μm (NeuN), 2 mm (GFAP and Iba1). D) Abl activity ELISAs of AblPP/tTA and ArgPP/tTA mouse cortex. Average picomoles of Abltide phosphorylated per kinase reaction, n = 4 per timepoint. E) Arg is expressed in neuronal cell bodies in the ArgPP/tTA mouse. Immunofluorescence of ArgPP/tTA mouse cortex with AR19 (red) and DAPI. F) ELISA analysis of tyrosine phosphorylated (4G10) tau/total (DA9) tau in ArgPP/tTA mice 12 and 24 weeks off dox. n = 4 for each timepoint. One-way ANOVA with Dunnett’s post-test was used to calculate whether ArgPP/tTA mice showed significant increases in tau phosphorlylation when compared to control mice. No significant differences in tau phosphorylation were found.
Similar articles
- Neuronal c-Abl activation leads to induction of cell cycle and interferon signaling pathways.
Schlatterer SD, Suh HS, Conejero-Goldberg C, Chen S, Acker CM, Lee SC, Davies P. Schlatterer SD, et al. J Neuroinflammation. 2012 Aug 31;9:208. doi: 10.1186/1742-2094-9-208. J Neuroinflammation. 2012. PMID: 22938163 Free PMC article. - Corticotrophin releasing factor accelerates neuropathology and cognitive decline in a mouse model of Alzheimer's disease.
Dong H, Murphy KM, Meng L, Montalvo-Ortiz J, Zeng Z, Kolber BJ, Zhang S, Muglia LJ, Csernansky JG. Dong H, et al. J Alzheimers Dis. 2012;28(3):579-92. doi: 10.3233/JAD-2011-111328. J Alzheimers Dis. 2012. PMID: 22045495 Free PMC article. - Characterization of neuronal and astroglial responses to ER stress in the hippocampal CA1 area in mice following transient forebrain ischemia.
Osada N, Kosuge Y, Ishige K, Ito Y. Osada N, et al. Neurochem Int. 2010 Aug;57(1):1-7. doi: 10.1016/j.neuint.2010.03.017. Epub 2010 Mar 31. Neurochem Int. 2010. PMID: 20362024 - c-Abl in neurodegenerative disease.
Schlatterer SD, Acker CM, Davies P. Schlatterer SD, et al. J Mol Neurosci. 2011 Nov;45(3):445-52. doi: 10.1007/s12031-011-9588-1. Epub 2011 Jul 5. J Mol Neurosci. 2011. PMID: 21728062 Free PMC article. Review. - Transgenic mouse models of Alzheimer's disease.
Bornemann KD, Staufenbiel M. Bornemann KD, et al. Ann N Y Acad Sci. 2000 Jun;908:260-6. doi: 10.1111/j.1749-6632.2000.tb06653.x. Ann N Y Acad Sci. 2000. PMID: 10911965 Review.
Cited by
- Clinically Precedented Protein Kinases: Rationale for Their Use in Neurodegenerative Disease.
Benn CL, Dawson LA. Benn CL, et al. Front Aging Neurosci. 2020 Sep 2;12:242. doi: 10.3389/fnagi.2020.00242. eCollection 2020. Front Aging Neurosci. 2020. PMID: 33117143 Free PMC article. Review. - Abl2 Kinase Differentially Regulates iGluRs Current Activity and Synaptic Localization.
Kabirova M, Reichenstein M, Borovok N, Sheinin A, Gorobets D, Michaelevski I. Kabirova M, et al. Cell Mol Neurobiol. 2023 Aug;43(6):2785-2799. doi: 10.1007/s10571-023-01317-9. Epub 2023 Jan 23. Cell Mol Neurobiol. 2023. PMID: 36689065 - Multikinase Abl/DDR/Src Inhibition Produces Optimal Effects for Tyrosine Kinase Inhibition in Neurodegeneration.
Fowler AJ, Hebron M, Missner AA, Wang R, Gao X, Kurd-Misto BT, Liu X, Moussa CE. Fowler AJ, et al. Drugs R D. 2019 Jun;19(2):149-166. doi: 10.1007/s40268-019-0266-z. Drugs R D. 2019. PMID: 30919310 Free PMC article. - A case of neurocognitive deficit strongly related to dasatinib therapy.
Chamoun K, Rabinovich E, Baer L, Fastenau P, Lima M. Chamoun K, et al. Hematol Transfus Cell Ther. 2020 Jan-Mar;42(1):80-82. doi: 10.1016/j.htct.2019.01.003. Epub 2019 Apr 23. Hematol Transfus Cell Ther. 2020. PMID: 31053490 Free PMC article. No abstract available. - Inverse relationship between Alzheimer's disease and cancer, and other factors contributing to Alzheimer's disease: a systematic review.
Shafi O. Shafi O. BMC Neurol. 2016 Nov 22;16(1):236. doi: 10.1186/s12883-016-0765-2. BMC Neurol. 2016. PMID: 27875990 Free PMC article. Review.
References
- Alzheimer’s Association. Alzheimer’s disease facts and figures. Alzheimers Dement. 2010;6:158–194. - PubMed
- Rojo LE, Fernandez JA, Maccioni AA, Jimenez JM, Maccioni RB. Neuroinflammation: implications for the pathogenesis and molecular diagnosis of Alzheimer’s disease. Arch Med Res. 2008;39:1–16. - PubMed
- Sheng JG, Mrak RE, Griffin WS. Neuritic plaque evolution in Alzheimer’s disease is accompanied by transition of activated microglia from primed to enlarged to phagocytic forms. Acta Neuropathol. 1997;94:1–5. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 AG022102/AG/NIA NIH HHS/United States
- NIMH38623/PHS HHS/United States
- T32GM007288/GM/NIGMS NIH HHS/United States
- T32 GM007288/GM/NIGMS NIH HHS/United States
- R37 AG022102/AG/NIA NIH HHS/United States
- R37 AG022102-10/AG/NIA NIH HHS/United States
- AG022102/AG/NIA NIH HHS/United States
- R37 AG022102-09/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous