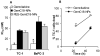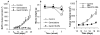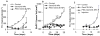In vitro and in vivo anti-tumor activities of a gemcitabine derivative carried by nanoparticles - PubMed (original) (raw)
Comparative Study
In vitro and in vivo anti-tumor activities of a gemcitabine derivative carried by nanoparticles
Brian R Sloat et al. Int J Pharm. 2011.
Abstract
Gemcitabine (Gemzar(®)) is the first line treatment for pancreatic cancer and often used in combination therapy for non-small cell lung, ovarian, and metastatic breast cancers. Although extremely toxic to a variety of tumor cells in culture, the clinical outcome of gemcitabine treatment still needs improvement. In the present study, a new gemcitabine nanoparticle formulation was developed by incorporating a previously reported stearic acid amide derivative of gemcitabine into nanoparticles prepared from lecithin/glyceryl monostearate-in-water emulsions. The stearoyl gemcitabine nanoparticles were cytotoxic to tumor cells in culture, although it took a longer time for the gemcitabine in the nanoparticles to kill tumor cells than for free gemcitabine. In mice with pre-established model mouse or human tumors, the stearoyl gemcitabine nanoparticles were significantly more effective than free gemcitabine in controlling the tumor growth. PEGylation of the gemcitabine nanoparticles with polyethylene glycol (2000) prolonged the circulation of the nanoparticles in blood and increased the accumulation of the nanoparticles in tumor tissues (>6-fold), but the PEGylated and un-PEGylated gemcitabine nanoparticles showed similar anti-tumor activity in mice. Nevertheless, the nanoparticle formulation was critical for the stearoyl gemcitabine to show a strong anti-tumor activity. It is concluded that for the gemcitabine derivate-containing nanoparticles, cytotoxicity data in culture may not be used to predict their in vivo anti-tumor activity, and this novel gemcitabine nanoparticle formulation has the potential to improve the clinical outcome of gemcitabine treatment.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures
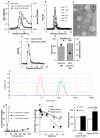
Fig. 1. Preparation and characterization of GemC18-NPs
(A). In GPC, GemC18-free NPs (○) and GemC18-NPs (□) eluted about two fractions earlier than GemC18 in Tween 20 micelles (□). The concentration of the GemC18 in the micelles and GemC18-NPs was 100 μg/mL. (B). Gel permeation chromatographs of GemC18-NPs prepared with 0, 0.1, 0.5, 1, 2.5, and 5 mg/mL of GemC18. In A & B, gemcitabine was measured at 248 nm. (C). TEM micrograph of the GemC18-NPs (with 5 mg/mL of GemC18). (D). Chromatographs of GemC18-NPs (●) and PEGylated GemC18-NPs (△) prepared with 5 mg/mL of GemC18. (E). The size and zeta potential of the GemC18-NPs and the PEG-GemC18-NPs (F). The dynamic light scattering spectra of the GemC18-in-Tween 20 micelles (left), GemC18-NPs, and PEG-GemC18-NPs (far right) overlaid. (G). The release of the GemC18 from the GemC18-NPs (●) or PEG-GemC18-NPs (△). (H). The release or hydrolysis of the gemcitabine from the GemC18-NPs when incubated in PBS, mouse serum, or human serum (values in the Y-axis are natural log product). (I). The size of the GemC18-NPs and PEG-GemC18-NPs after 30 min of incubation at 37°C in FBS in normal saline. Except in C and F, all data presented were the mean from at least 3 independent determinations. Standard deviations were not included in some figures for clarity.
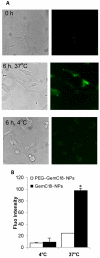
Fig. 2. The uptake of GemC18-NPs by TC-1 tumor cells in culture
(A). Fluorescence micrographs. Cells were incubated with fluorescein-labeled GemC18-NPs for 6 h at 37°C or 4°C and observed under a bright-field microscope (left panel) or a fluorescence microscope (right panel). Photos were taken at 20 × magnification. (B). Comparison of the uptakes of PEGylated and un-PEGylated GemC18-NPs. * p < 0.001, PEG-GemC18-NPs vs. GemC18-NPs at 37°C.
Fig. 3. GemC18-NPs were cytotoxic to tumor cells in culture
(A). The IC50 values of gemcitabine, GemC18-NPs, and PEG-GemC18-NPs in TC-1 and BxPC-3 cells. Cells were incubated with gemcitabine HCl or nanoparticles for 48 h. * For both cell lines, p < 0.05, Gemcitabine vs. GemC18-NPs. (B). It took the GemC18-NPs a longer time than the gemcitabine HCl to kill tumor cells. TC-1 cells were incubated with gemcitabine HCl or GemC18-NPs at 28.7 nM for 24 or 48 h, and the % of surviving cells was determined. Data are mean ± S.D. (n = 3-4).
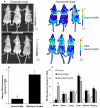
Fig. 4. In vivo and ex vivo imaging of GemC18-NPs and PEG-GemC18-NPs
(A). IVIS images of athymic mice 24 h after injection of fluorescein-labeled GemC18-NPs or PEG-GemC18-NPs. (B). Relative fluorescence intensity values in BxPC-3 tumors (circular ROI in A). a p = 0.0006, GemC18-NPs vs. PEG-GemC18-NPs. (C). Tissue distribution of fluorescein-labeled GemC18-NPs and PEG-GemC18-NPs 24 h after injection. b GemC18-NPs vs. PEG-GemC18-NPs, p = 0.003, 0.021, and 0.002 for blood, liver, and spleen, respectively.
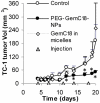
Fig. 5. In vivo anti-tumor activity of the GemC18-NPs against TC-1 tumors in C57BL/6 mice
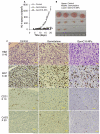
(A). TC-1 tumor growth curves in C57BL/6 mice. Tumor cells were implanted on day 0. On days 4 and 13, mice (n = 4) were i.v. injected with GemC18-NPs, gemcitabine HCl, or sterile mannitol. Data reported are mean ± S.D. * The values of Gemcitabine and GemC18-NPs were different starting from day 8 (p < 0.05). This experiment was repeated 3 times to confirm the anti-tumor activity of the GemC18-NPs, and similar result was obtained. (B). Photographs of TC-1 tumors 21 days after tumor cell injection. (C). (Immuno)histograms of TC-1 tumors after treatment with gemcitabine HCl or GemC18-NPs. CAS3, caspase 3 staining.
Fig. 6. In vivo anti-tumor activity of GemC18-NPs against BxPC-3 tumors in athymic mice
(A). BxPC-3 tumor growth curves. Tumor cells were seeded on day 0, and mice were i.v. injected on days 6 and 19. (B). Average weight of BxPC-3 tumor-bearing mice after different treatments. * p = 0.0007 (ANOVA on week 3). (C). GemC18-free nanoparticles lack anti-tumor activity. BxPC-3 cells were seeded on day 0, and mice were i.v. injected once on day 4. NPs, GemC18-free nanoparticles.
Fig. 7. Comparison of the in vivo anti-tumor activities of GemC18-NPs and PEGylated GemC18-NPs
(A). TC-1 tumors in C57BL/6 mice. Mice (n = 5-7) were injected (i.v.) with GemC18-NPs or PEG-GemC18-NPs once (1 mg GemC18 per mouse). (B). BxPC-3 tumors in athymic mice. Mice (n = 5) were injected (i.v.) with GemC18-NPs or PEG-GemC18-NPs 3 times (days 0, 12, and 21). In A&B, tumor sizes were reported starting from the day of the injection of the nanoparticles. (C). TC-1 tumors in C57BL/6 mice. The nanoparticles were injected peritumorally (0.25 mg of GemC18 per mouse at each injection). Data shown are mean ± S.E.M. Statistical analysis did not reveal any differences between the GemC18-NPs and PEG-GemC18-NPs in A, B, and C.
Fig. 8. The GemC18-NPs were more effective than the GemC18-in-Tween 20 micelles
PEG-GemC18-NPs or GemC18-in-Tween 20 micelles were injected twice a week for 5 times (150 μg of GemC18 per mouse). *, p < 0.05, PEG-GemC18-NPs vs. GemC18-in-Tween 20 micelles starting on day 12. Data shown are mean ± S.E.M.
Similar articles
- Preparation of intravenous injection nanoformulation of VESylated gemcitabine by co-assembly with TPGS and its anti-tumor activity in pancreatic tumor-bearing mice.
Xu Y, Meng H, Du F, Lu W, Liu S, Huang J, Yu J. Xu Y, et al. Int J Pharm. 2015 Nov 30;495(2):792-7. doi: 10.1016/j.ijpharm.2015.09.030. Epub 2015 Sep 26. Int J Pharm. 2015. PMID: 26410754 - PEGylated liposomal Gemcitabine: insights into a potential breast cancer therapeutic.
Papa AL, Sidiqui A, Balasubramanian SU, Sarangi S, Luchette M, Sengupta S, Harfouche R. Papa AL, et al. Cell Oncol (Dordr). 2013 Dec;36(6):449-57. doi: 10.1007/s13402-013-0146-4. Epub 2013 Oct 1. Cell Oncol (Dordr). 2013. PMID: 24081907 - Diels Alder-mediated release of gemcitabine from hybrid nanoparticles for enhanced pancreatic cancer therapy.
Oluwasanmi A, Al-Shakarchi W, Manzur A, Aldebasi MH, Elsini RS, Albusair MK, Haxton KJ, Curtis ADM, Hoskins C. Oluwasanmi A, et al. J Control Release. 2017 Nov 28;266:355-364. doi: 10.1016/j.jconrel.2017.09.027. Epub 2017 Sep 22. J Control Release. 2017. PMID: 28943195 - Magnetic poly epsilon-caprolactone nanoparticles containing Fe3O4 and gemcitabine enhance anti-tumor effect in pancreatic cancer xenograft mouse model.
Gang J, Park SB, Hyung W, Choi EH, Wen J, Kim HS, Shul YG, Haam S, Song SY. Gang J, et al. J Drug Target. 2007 Jul;15(6):445-53. doi: 10.1080/10611860701453901. J Drug Target. 2007. PMID: 17613663 - Gemcitabine-loaded liposomes: rationale, potentialities and future perspectives.
Federico C, Morittu VM, Britti D, Trapasso E, Cosco D. Federico C, et al. Int J Nanomedicine. 2012;7:5423-36. doi: 10.2147/IJN.S34025. Epub 2012 Nov 1. Int J Nanomedicine. 2012. PMID: 23139626 Free PMC article. Review.
Cited by
- Stearoyl gemcitabine nanoparticles overcome obesity-induced cancer cell resistance to gemcitabine in a mouse postmenopausal breast cancer model.
De Angel RE, Blando JM, Hogan MG, Sandoval MA, Lansakara-P DS, Dunlap SM, Hursting SD, Cui Z. De Angel RE, et al. Cancer Biol Ther. 2013 Apr;14(4):357-64. doi: 10.4161/cbt.23623. Epub 2013 Jan 28. Cancer Biol Ther. 2013. PMID: 23358472 Free PMC article. - Prevention of nodal metastases in breast cancer following the lymphatic migration of paclitaxel-loaded expansile nanoparticles.
Liu R, Gilmore DM, Zubris KA, Xu X, Catalano PJ, Padera RF, Grinstaff MW, Colson YL. Liu R, et al. Biomaterials. 2013 Feb;34(7):1810-9. doi: 10.1016/j.biomaterials.2012.11.038. Epub 2012 Dec 8. Biomaterials. 2013. PMID: 23228419 Free PMC article. - Microneedle-mediated transcutaneous immunization with plasmid DNA coated on cationic PLGA nanoparticles.
Kumar A, Wonganan P, Sandoval MA, Li X, Zhu S, Cui Z. Kumar A, et al. J Control Release. 2012 Oct 28;163(2):230-9. doi: 10.1016/j.jconrel.2012.08.011. Epub 2012 Aug 19. J Control Release. 2012. PMID: 22921518 Free PMC article. - A nanoparticle depot formulation of 4-(N)-stearoyl gemcitabine shows a strong anti-tumour activity.
Zhu S, Li X, Lansakara-P DS, Kumar A, Cui Z. Zhu S, et al. J Pharm Pharmacol. 2013 Feb;65(2):236-42. doi: 10.1111/j.2042-7158.2012.01599.x. Epub 2012 Oct 14. J Pharm Pharmacol. 2013. PMID: 23278691 Free PMC article. - Ciprofloxacin Derivative-Loaded Nanoparticles Synergize with Paclitaxel Against Type II Human Endometrial Cancer.
Naguib YW, Alhaj-Suliman SO, Wafa EI, Saha S, Ebeid K, Mohammed HHH, Abdel-Rahman SA, Abuo-Rahma GEA, Geary SM, Salem AK. Naguib YW, et al. Small. 2024 Oct;20(41):e2302931. doi: 10.1002/smll.202302931. Epub 2023 Jul 31. Small. 2024. PMID: 37525558
References
- Abbruzzese JL, Grunewald R, Weeks EA, Gravel D, Adams T, Nowak B, Mineishi S, Tarassoff P, Satterlee W, Raber MN, et al. A phase I clinical, plasma, and cellular pharmacology study of gemcitabine. J Clin Oncol. 1991;9:491–498. - PubMed
- Arias JL, Reddy LH, Couvreur P. Magnetoresponsive squalenoyl gemcitabine composite nanoparticles for cancer active targeting. Langmuir. 2008;24:7512–7519. - PubMed
- Arias JL, Reddy LH, Couvreur P. Polymeric nanoparticulate system augmented the anticancer therapeutic efficacy of gemcitabine. J Drug Target. 2009;17:586–598. - PubMed
- Bergman AM, Adema AD, Balzarini J, Bruheim S, Fichtner I, Noordhuis P, Fodstad O, Myhren F, Sandvold ML, Hendriks HR, Peters GJ. Antiproliferative activity, mechanism of action and oral antitumor activity of CP-4126, a fatty acid derivative of gemcitabine, in in vitro and in vivo tumor models. Invest New Drugs. 2010 Epub. - PMC - PubMed
- Bergman AM, Pinedo HM, Peters GJ. Determinants of resistance to 2′,2′-difluorodeoxycytidine (gemcitabine) Drug Resist Updat. 2002;5:19–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA135274/CA/NCI NIH HHS/United States
- R01 CA135274-05/CA/NCI NIH HHS/United States
- R01 CA135274-04/CA/NCI NIH HHS/United States
- R01 CA135274/CA/NCI NIH HHS/United States
- R01 CA135274-03S1/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources