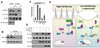Yap1 acts downstream of α-catenin to control epidermal proliferation - PubMed (original) (raw)
Yap1 acts downstream of α-catenin to control epidermal proliferation
Karin Schlegelmilch et al. Cell. 2011.
Abstract
During development and regeneration, proliferation of tissue-specific stem cells is tightly controlled to produce organs of a predetermined size. The molecular determinants of this process remain poorly understood. Here, we investigate the function of Yap1, the transcriptional effector of the Hippo signaling pathway, in skin biology. Using gain- and loss-of-function studies, we show that Yap1 is a critical modulator of epidermal stem cell proliferation and tissue expansion. Yap1 mediates this effect through interaction with TEAD transcription factors. Additionally, our studies reveal that α-catenin, a molecule previously implicated in tumor suppression and cell density sensing in the skin, is an upstream negative regulator of Yap1. α-catenin controls Yap1 activity and phosphorylation by modulating its interaction with 14-3-3 and the PP2A phosphatase. Together, these data identify Yap1 as a determinant of the proliferative capacity of epidermal stem cells and as an important effector of a "crowd control" molecular circuitry in mammalian skin.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
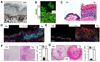
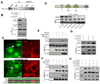
Figure 1. Activation of Yap1 leads to epidermal stem cell expansion
A, Yap1 expression (brown) indicated by immunohistochemical staining on E18.5 murine wild-type epidermis. B, Immunofluorescence staining for Yap1 shows a dynamic localization pattern in human keratinocytes that is cell density-dependent. C, H&E staining shows an abnormally thick epidermis and hyperkeratinization in adult Tg mouse skin after 8 days of doxycyclin (Dox) induction. D-E, Immunofluorescence analysis on frozen sections of adult epidermis shows an expansion of the proliferative Ki-67 positive compartment as well as the stem cell compartment (p63-positive, K5-positive) in Tg skin after 8 days of dox induction. Dashed line marks epidermal-dermal junction. F, Rhodamine B staining of primary mouse keratinocytes isolated from 8-day Dox-treated Tg mice, and cultured for 9 days on feeders, shows a significant expansion of the epidermal stem cell compartment demonstrated by a higher colony-forming efficiency in Tg skin. G, Rhodamine B staining and colony counts show a significant increase in the self-renewal capacity of colony-forming progenitors after Dox administration measured by serial replating assays (shown is passage 2, P2). * p< 0.05, **p value < 0.01. Scale bars represent 20 µm. See also Supplementary Figure S1A–C.
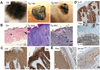
Figure 2. Activation of Yap1 leads to tumor formation
A, Gross morphology of Ctr and Tg grafts 14 and 42 days after beginning of Dox treatment. Note hyperkeratosis and stunted hair growth in Tg 14d graft and ulceration of skin and subcutaneous tumor mass in Tg 42d graft. B, H&E staining of Ctr and Tg grafts 9 and 42 days after Dox induction show an invasion of underlying dermis by invasive keratinocytes derived from the Tg graft. Arrow points out to neighboring normal Nude mouse epidermis. White dashed line indicates border of carcinoma to normal Nude dermis.C, Expression of cyto-keratin (CK) and keratin 6 (K6) in Tg grafts treated 9 days with Dox as shown by immunohistochemistry. D, Immunohistochemistry for Yap1 on Tg grafts with 9d and 42d Dox treatment reveals epithelial origin of the tumor. Arrow points at Nude mouse epidermis. E, Immunohistochemistry for vimentin (Vim) and integrin-β4 (β4-int) on Tg grafts with 42 days Dox induction. Scale bars represent 100 µm. See also Supplementary Figure S1D–H.
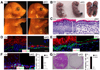
Figure 3. Yap1 is required for the maintenance of the proliferative capacity of epidermal stem cells
A, Gross morphology of representative control (Ctr) and cKO E18.5 embryos. Note absence of skin in distal limbs, eyes, and ears and overall thinner skin. B, Toluidine blue skin barrier assay in E18.5 mice reveal absence of epidermal barrier in limbs, ears, nose and mouth. C, H&E staining shows a decrease in epidermal thickness in cKO skin compared to the Ctr (indicated by bracket). Note the flat morphology of basal cells and disorganized epidermal architecture. Dashed line represents epidermal-dermal junction. D-E, Immunofluorescence on frozen E18.5 epidermis reveals signs of basal cell depletion in cKO skin. Note the reduction of keratin 10 (K10) negative, keratin 5 (K5) positive cells progenitor/stem cells juxatposed to the basement membrane (β4). Note background staining for K5 in stratum corneum of cKO. F, Marked reduction in number of proliferative basal cells in cKO limb skin as measured with an antibody against phospho-histone H3 (pH3). Results represent measurements of at least 5 different fields in 3 different animals. G, Reduction in the number of colony-forming progenitors in E18.5 skin. Rhodamine B stain and colony counts were performed at day eight after plating and are representative of two independent experiments. Data are presented as mean ± standard deviation (error bars). **p value < 0.01, *** p< 0.005. Scale bars represent 20 µm. See also Supplementary Figure S2A–F.
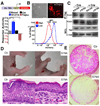
Figure 4. Yap1 function in the skin is mediated through TEAD factors
A, A HaCaT keratinocyte cell line carrying multimerized TEAD-binding sequences (TBS) upstream of a minimal promoter (minP) and an mCherry reporter is responsive to expression of Yap1-S127A, whereas RNAi KD of Yap1 downregulates reporter levels. Cells that have been transfected with a scrambled control-RNAi display reporter activity at basal levels. B, TBS reporter dynamics mimic Yap1 activity/localization. Top panel, TBS-mCherry shows a dynamic fluorescence pattern with keratinocytes at the edge of a growing colony expressing higher mCherry levels. Bottom panel, reporter signal decreases significantly at high cellular densities. C, Physical interaction between Yap1 and TEAD1 is virtually ablated in Adenovirus-Cre (+Cre) treated Yapfl/S79A embryonic fibroblasts. Immunoprecipitation (IP) was performed with an anti-Yap1 antibody followed by immunoblotting for TEAD1. Loading control β-tubulin (β-tub). D, Gross morphology of control (Ctr) and K14-Cre Yapfl/S79A E18.5 embryos. Note absence of skin in distal limbs and thinner skin with flat basal cells. E, Reduction in the number of colony-forming progenitors and proliferative potential in the skin of E18.5 K14-Cre Yapfl/S79A mice as demonstrated by colony assays stained with Rhodamine B. Data are presented as mean ± standard deviation (error bars), **p < 0.01, ***p < 0.005. Scale bars represent 20 µm. See also Supplementary Figure S3A–C.
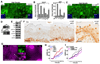
Figure 5. α-Catenin interacts with Yap1 and 14-3-3
A, Confluent HaCaT cell lysates (Input) were used for an IP with an antibody against Yap1 or Pu.1 (Ctrl) and IP and post-IP material blotted with an antibody for α-catenin. B, 293T cells were co-transfected with the indicated plasmids followed by immunoprecipitation (IP) with either GST or FLAG-beads. Co-IPs demonstrate an association between full-length Yap1 and α-catenin and a largely diminished association between α-catenin and the YapS127A mutant. IB: Immunoblotting, WCL: Whole cell lysate. C, Calcium-induced differentiation in primary human keratinocytes leads to translocation of Yap1 into membrane regions that colocalize with α-catenin. scale bars = 10uM. D, Characterization of Yap1 domains important for the interaction with α-catenin.E–F, In vitro pulldown of recombinant Yap1 and α-catenin in the presence or absence of 14-3-3. G, In vitro pulldown of recombinant 〈-catenin and 14-3-3 in the presence of phosphorylated recombinant Yap1. H, Immunoprecipitation of Yap1 and α-catenin demonstrates that the biochemical interaction is dependent on 14-3-3. Scale bars depicted are 10 µm. See also Supplementary Figure S4A–E, S5A–B and Table S1.
Figure 6. α-Catenin regulates Yap1 localization and activity
A, Disruption of AJs in high-confluent keratinocytes with EGTA results in Yap1 nuclear localization after 1-minute of treatment. B, Knockdown (KD) of AJ proteins in either HaCaT or 293T cells carrying the TBS-reporter. KD of NF2 is shown as a positive control. C, KD of α-cat in confluent keratinocytes leads to Yap1 nuclear localization, and interaction with its nuclear partner Tead1 (D). E, siRNA-mediated depletion of α-cat in high density HaCaT cultures leads to loss of Yap phosporylation at serine 127 (pYap), and unchanged levels of activated Mst1/2 (pMst) or Lats1/2 (pLats). F, Immunohistochemistry for Yap1 in Ctrl and K14-Cre conditional α-catenin mutant (cKO) E18.5 epidermis. Note enhanced nuclear staining in basal and suprabasal cells of cKO mice. G, Immunofluorescence detection of Yap1 (purple) localization in low-density keratinocytes ectopically expressing GST-α-cat (green). Keratinocytes overexpressing α-cat show Yap1 localization to sites of cell-cell contact, whereas untransfected cells show nuclear staining and an absence of Yap1 staining at the cell membrane. H, Stable-knockdown of α-catenin (α-cat-KD) promotes hyperproliferation in HaCaT cells, however doxycycline (dox)-induced KD of Yap1 in α-cat-KD cells slows down the rate of cell proliferation to control (Ctr) levels. I, Ectopic expression of α-cat in a HaCaT cells suppresses cell proliferation (α-catOE). This growth inhibition is rescued by the expression of a Dox-inducible Yap1S127A mutant (Yap1S127A+Dox). Data are mean ± standard deviation (error bars), ***p < 0.001. Scale bars are 20 µm (A, C), and 10 µm (F), 5 µm (G). See also Supplementary Figure S5C–H and S6A–E.
Figure 7. α-Catenin binding prevents Yap1 dephosphorylation and activation
A, PP2Ac associates with Yap1 in α-catenin-depleted HaCaT cells. The association of Yap1 and 14-3-3 is diminished in the absence of α-catenin. B, Yap1 is dephosphorylated by PP2Ac in vitro. Different concentrations of purified PP2A was incubated with Sf9-purified recombinant Yap1 for 30 minutes. C, siRNA KD of α-catenin increases TBS-reporter activation and the dephosphorylation of Yap1. Simultanious KD of α-catenin and PP2Ac suppresses TBS-reporter transcriptional activity and and increase in Yap1 phosphorylation. D, Western blot analysis of phospo-Yap levels following an in vitro dephosphorylation competition assay of Yap1 by PP2Ac in the presence of recombinant 14-3-3 and α-catenin. E, Schematic model of density-dependent and α-catenin-mediated Yap1 activation. Data are presented as mean ± standard deviation (error bars), **p < 0.01, ***p < 0.001. See also Supplementary Figure S7.
Similar articles
- αE-catenin inhibits a Src-YAP1 oncogenic module that couples tyrosine kinases and the effector of Hippo signaling pathway.
Li P, Silvis MR, Honaker Y, Lien WH, Arron ST, Vasioukhin V. Li P, et al. Genes Dev. 2016 Apr 1;30(7):798-811. doi: 10.1101/gad.274951.115. Epub 2016 Mar 24. Genes Dev. 2016. PMID: 27013234 Free PMC article. - α-catenin is a tumor suppressor that controls cell accumulation by regulating the localization and activity of the transcriptional coactivator Yap1.
Silvis MR, Kreger BT, Lien WH, Klezovitch O, Rudakova GM, Camargo FD, Lantz DM, Seykora JT, Vasioukhin V. Silvis MR, et al. Sci Signal. 2011 May 24;4(174):ra33. doi: 10.1126/scisignal.2001823. Sci Signal. 2011. PMID: 21610251 Free PMC article. - Mammalian Tead proteins regulate cell proliferation and contact inhibition as transcriptional mediators of Hippo signaling.
Ota M, Sasaki H. Ota M, et al. Development. 2008 Dec;135(24):4059-69. doi: 10.1242/dev.027151. Epub 2008 Nov 12. Development. 2008. PMID: 19004856 - 14-3-3σ regulates keratinocyte proliferation and differentiation by modulating Yap1 cellular localization.
Sambandam SAT, Kasetti RB, Xue L, Dean DC, Lu Q, Li Q. Sambandam SAT, et al. J Invest Dermatol. 2015 Jun;135(6):1621-1628. doi: 10.1038/jid.2015.42. Epub 2015 Feb 10. J Invest Dermatol. 2015. PMID: 25668240 Free PMC article. - The Hippo Pathway as Drug Targets in Cancer Therapy and Regenerative Medicine.
Nagashima S, Bao Y, Hata Y. Nagashima S, et al. Curr Drug Targets. 2017;18(4):447-454. doi: 10.2174/1389450117666160112115641. Curr Drug Targets. 2017. PMID: 26758663 Review.
Cited by
- ETS1 Expression in Diabetic Foot Ulcers: Implications for Fibroblast Phenotype and Wound Healing Through the PP2A/YAP Pathway.
Yi W, Bao Q, Xu D, Long C, Fang R, Cheng W, Song J, Feng H. Yi W, et al. J Inflamm Res. 2024 Oct 16;17:7373-7388. doi: 10.2147/JIR.S477470. eCollection 2024. J Inflamm Res. 2024. PMID: 39429853 Free PMC article. - The subcortical maternal complex modulates the cell cycle during early mammalian embryogenesis via 14-3-3.
Han Z, Wang R, Chi P, Zhang Z, Min L, Jiao H, Ou G, Zhou D, Qin D, Xu C, Gao Z, Qi Q, Li J, Lu Y, Wang X, Chen J, Yu X, Hu H, Li L, Deng D. Han Z, et al. Nat Commun. 2024 Oct 15;15(1):8887. doi: 10.1038/s41467-024-53277-3. Nat Commun. 2024. PMID: 39406751 Free PMC article. - Versatility of 14-3-3 proteins and their roles in bone and joint-related diseases.
Zhou R, Hu W, Ma PX, Liu CJ. Zhou R, et al. Bone Res. 2024 Oct 15;12(1):58. doi: 10.1038/s41413-024-00370-4. Bone Res. 2024. PMID: 39406741 Free PMC article. Review. - The Multifaceted Roles of Hippo-YAP in Cardiovascular Diseases.
Wu H, Che YN, Lan Q, He YX, Liu P, Chen MT, Dong L, Liu MN. Wu H, et al. Cardiovasc Toxicol. 2024 Dec;24(12):1410-1427. doi: 10.1007/s12012-024-09926-6. Epub 2024 Oct 4. Cardiovasc Toxicol. 2024. PMID: 39365552 Review. - A feedback loop between plakophilin 4 and YAP signaling regulates keratinocyte differentiation.
Müller L, Gutschner T, Hatzfeld M. Müller L, et al. iScience. 2024 Aug 19;27(9):110762. doi: 10.1016/j.isci.2024.110762. eCollection 2024 Sep 20. iScience. 2024. PMID: 39286493 Free PMC article.
References
- Baumgartner R, Poernbacher I, Buser N, Hafen E, Stocker H. The WW domain protein Kibra acts upstream of Hippo in Drosophila. Dev Cell. 2010;18:309–316. - PubMed
- Camargo FD, Gokhale S, Johnnidis JB, Fu D, Bell GW, Jaenisch R, Brummelkamp TR. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol. 2007;17:2054–2060. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases