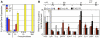A transient definitive erythroid lineage with unique regulation of the β-globin locus in the mammalian embryo - PubMed (original) (raw)
A transient definitive erythroid lineage with unique regulation of the β-globin locus in the mammalian embryo
Kathleen E McGrath et al. Blood. 2011.
Abstract
A transient erythromyeloid wave of definitive hematopoietic progenitors (erythroid/myeloid progenitors [EMPs]) emerges in the yolk sac beginning at embryonic day 8.25 (E8.25) and colonizes the liver by E10.5, before adult-repopulating hematopoietic stem cells. At E11.5, we observe all maturational stages of erythroid precursors in the liver and the first definitive erythrocytes in the circulation. These early fetal liver erythroblasts express predominantly adult β-globins and the definitive erythroid-specific transcriptional modifiers c-myb, Sox6, and Bcl11A. Surprisingly, they also express low levels of "embryonic" βH1-, but not εy-, globin transcripts. Consistent with these results, RNA polymerase and highly modified histones are found associated with βH1- and adult globin, but not εy-globin, genes. E11.5 definitive proerythroblasts from mice transgenic for the human β-globin locus, like human fetal erythroblasts, express predominately human γ-, low β-, and no ε-globin transcripts. Significantly, E9.5 yolk sac-derived EMPs cultured in vitro have similar murine and human transgenic globin expression patterns. Later liver proerythroblasts express low levels of γ-globin, while adult marrow proerythroblasts express only β-globin transcripts. We conclude that yolk sac-derived EMPs, the first of 2 origins of definitive erythropoiesis, express a unique pattern of globin genes as they generate the first definitive erythrocytes in the liver of the mammalian embryo.
Figures
Figure 1
Onset of definitive erythropoiesis by E11.5. (A) Imaging flow cytometry of E11.5 blood. Definitive RBCs (top) have higher mean levels of Ter119 staining and lighter brightfield intensity then cocirculating primitive erythroid cells (bottom). (B) Percent of total erythroid (Ter119-positive) cells that are definitive in fetal blood are plotted by embryonic day (note log scale). Low but consistent numbers of definitive erythroid cells are seen by E11.5. (C) Median diameter of definitive erythroid cells demonstrates early definitive RBC size is consistent with later fetal RBC size and different from primitive or adult definitive erythroid cells. (D) Imaging flow cytometry demonstrated all stages of definitive erythroblasts in the E11.5 liver. Maturational stages were determined by combined morphologic and fluorescent parameters including cell and nuclear size, RNA (thiazole orange) staining and mean intensity of Ter119 and DNA (DRAQ5) staining. (E) Absolute numbers of staged erythroblasts per liver during fetal development. Inset is a smaller scale plot of E11.5 data. Error bars are SEM, n = 3 or 4.
Figure 2
βH1-globin, but not εy-globin, transcripts are present in the early fetal liver. Neighboring sections of E12.5 liver were hybridized to radioactive probes (exposed grains indicating hybridized probe are colorized red). β1-globin is expressed predominantly in liver (fl) cells, while εy-globin is expressed in circulating primitive erythroid cells (fb). βH1-globin is expressed in low levels in primitive erythroid cells as expected, but also more highly expressed in a subset of cells in the liver. Size bar equals 100 μm. Images were processed as described in “In situ hybridization.”
Figure 3
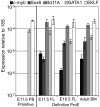
βH1-globin expression in fetal liver definitive erythroid cells. (A) Levels of εy-, βH1-, and β1-globin transcripts were determined by qPCR and graphed as a percentage of total β-globin transcripts. Circulating E11.5 primitive erythroid cells (PB) express high levels of εy- and βH1-globin. Proerythroblasts (Ter119lo, c-kit+) from late fetal liver and adult BM express β1-globin and no embryonic globins. While E11.5 liver proerythroblasts express a unique combination of β-globins with both βH1- and β1- but little if any εy-globin. Error bars are SEM of 3 samples. (B) RNA Pol II occupancy at the β-globin locus was determined by ChIP. The murine β-globin locus is depicted to scale above the graph with the probes used indicated by short dashes. Significantly higher levels of RNA Pol II were found associated the βH1-globin promoter than the εy-globin promoter in E11.5 fetal liver cells (P < .007) based on 1-way ANOVA analysis. Error bars represent SEM of 3 samples.
Figure 4
βH1-globin expression is associated with yolk sac–derived definitive hematopoietic progenitors. (A) E9.5 yolk sac progenitors (c-kithi, CD41hi) cultured in a 2-step erythroid differentiation assay express βH1-globin as they mature and switch to predominantly β1-globin expression. SEM of 3 experiments is indicated by error bars. (B) Globin expression of cells derived from individual BFU-E–derived cultures expressing βH1-globin are only found in the yolk sac and early fetal liver. Pale red BFU-E colonies at day 2 to 4 methylcellulose cultures were analyzed individually by qPCR for expression of εy-, βH1-, and β1-globin. Proportions of colonies with βH1-globin expression over the total colonies analyzed are shown.
Figure 5
Early fetal liver erythropoiesis associated with c-myb, Sox6, and Bcl11A erythroid transcription factors. Expression of common erythroid transcription factors (GATA-1, EKLF) and those uniquely expressed in definitive erythroid lineages (c-myb, Sox6, and Bcl11A) in primitive erythroid cells from peripheral blood (PB) and in definitive proerythroblasts sorted from fetal liver (FL) and BM was determined by qPCR. Error bars indicate SEM and n = 3.
Figure 6
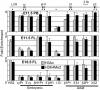
Unique chromatin modification patterns of the β-globin locus in primitive, EMP-derived fetal, and HSC-derived adult erythropoiesis. ChIP of modified histones (H3Ac or H3K4Me2) in the β-globin locus. Locations of amplimers are indicated by black bars in the diagram above and enrichments graphed in order below. Enrichment of H3Ac and H3K4Me2 was found in primitive erythroid cells from E11.5 peripheral blood (PB) in both embryonic and adult globin genes regions but only in adult genes in later fetal liver (E16.5 FL). E11.5 fetal liver had enrichment of modified histones over the adult genes and the βH1-globin gene but not εy-globin. Error bars represent the SEM and n = 3.
Figure 7
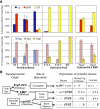
Human γ-globin is highly expressed in βH1-globin–expressing definitive proerythroblasts. (A) Mice with a single copy of the human β-globin locus were assayed by qPCR for gene expression of murine (top) and human (bottom) β-globin genes. Levels of globin transcripts are graphed as the percentage of total β-globin message measured from that locus. The left 3 samples are from primitive erythroid cells sorted from fetal blood (Ter119+ c-kit−). The middle 3 samples are from sorted definitive proerythroblasts (Ter119lo c-kit+) from fetal liver or BM. The right 2 samples are cultured E9.5 yolk sac EMP in erythroid maturation media. Error bars derived from the SEM of 3 independent experiments. (B) Three erythroid lineages emerge during mammalian embryogenesis. Two of these lineages, primitive erythroid and EMP-definitive erythroid, originate in the yolk sac. The AGM and other sites generate the HSC that colonize the liver and eventually the BM. Primitive erythroid precursors mature in the circulation, while EMP- and HSC-derived BFU-E mature in the liver of the fetus. Different patterns of β-globin expression in the mouse, human, and of the transgenic human genes in mice characterize these different forms of erythropoiesis (Figures 3, 7A).,– Genes are presented in their order within the β-globin locus. Grayed gene names signify no expression in that lineage. †While the yolk sac is the only reported site of EMP emergence precirculation, EMP may also be produced elsewhere and obscured from detection by the presence of EMP in circulating blood. *Maturational globin switching is observed in the first 2 lineages in mouse where βH1-globin or transgenic human γ-globin decrease in prevalence as erythroid precursors mature. ⋀β-globin is the major β-globin expressed in adult humans, however, rare γ-globin–expressing F cells are also found in the circulation.
Similar articles
- "Maturational" globin switching in primary primitive erythroid cells.
Kingsley PD, Malik J, Emerson RL, Bushnell TP, McGrath KE, Bloedorn LA, Bulger M, Palis J. Kingsley PD, et al. Blood. 2006 Feb 15;107(4):1665-72. doi: 10.1182/blood-2005-08-3097. Epub 2005 Nov 1. Blood. 2006. PMID: 16263786 Free PMC article. - Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse.
Palis J, Robertson S, Kennedy M, Wall C, Keller G. Palis J, et al. Development. 1999 Nov;126(22):5073-84. doi: 10.1242/dev.126.22.5073. Development. 1999. PMID: 10529424 - Induction of adult levels of β-globin in human erythroid cells that intrinsically express embryonic or fetal globin by transduction with KLF1 and BCL11A-XL.
Trakarnsanga K, Wilson MC, Lau W, Singleton BK, Parsons SF, Sakuntanaga P, Kurita R, Nakamura Y, Anstee DJ, Frayne J. Trakarnsanga K, et al. Haematologica. 2014 Nov;99(11):1677-85. doi: 10.3324/haematol.2014.110155. Epub 2014 Aug 8. Haematologica. 2014. PMID: 25107887 Free PMC article. - Developmental niches for embryonic erythroid cells.
Isern J, Fraser ST, He Z, Baron MH. Isern J, et al. Blood Cells Mol Dis. 2010 Apr 15;44(4):207-8. doi: 10.1016/j.bcmd.2010.02.008. Epub 2010 Feb 24. Blood Cells Mol Dis. 2010. PMID: 20181503 Free PMC article. Review. - Primitive erythropoiesis in the mammalian embryo.
Palis J, Malik J, McGrath KE, Kingsley PD. Palis J, et al. Int J Dev Biol. 2010;54(6-7):1011-8. doi: 10.1387/ijdb.093056jp. Int J Dev Biol. 2010. PMID: 20711979 Review.
Cited by
- The Coup-TFII orphan nuclear receptor is an activator of the γ-globin gene.
Fugazza C, Barbarani G, Elangovan S, Marini MG, Giolitto S, Font-Monclus I, Marongiu MF, Manunza L, Strouboulis J, Cantù C, Gasparri F, Barabino SML, Nakamura Y, Ottolenghi S, Moi P, Ronchi AE. Fugazza C, et al. Haematologica. 2021 Feb 1;106(2):474-482. doi: 10.3324/haematol.2019.241224. Haematologica. 2021. PMID: 32107331 Free PMC article. - The erythroblastic island as an emerging paradigm in the anemia of inflammation.
Hom J, Dulmovits BM, Mohandas N, Blanc L. Hom J, et al. Immunol Res. 2015 Dec;63(1-3):75-89. doi: 10.1007/s12026-015-8697-2. Immunol Res. 2015. PMID: 26376896 Free PMC article. Review. - Development and differentiation of the erythroid lineage in mammals.
Barminko J, Reinholt B, Baron MH. Barminko J, et al. Dev Comp Immunol. 2016 May;58:18-29. doi: 10.1016/j.dci.2015.12.012. Epub 2015 Dec 19. Dev Comp Immunol. 2016. PMID: 26709231 Free PMC article. Review. - Macrophage protease-activated receptor 2 regulates fetal liver erythropoiesis in mice.
Saffarzadeh M, Grunz K, Nguyen TS, Lee YK, Kitano M, Danckwardt S, Rodrigues CDS, Weiler H, Reyda S, Ruf W. Saffarzadeh M, et al. Blood Adv. 2020 Nov 24;4(22):5810-5824. doi: 10.1182/bloodadvances.2020003299. Blood Adv. 2020. PMID: 33232477 Free PMC article. - Common developmental pathway for primitive erythrocytes and multipotent hematopoietic progenitors in early mouse development.
Yamane T, Washino A, Yamazaki H. Yamane T, et al. Stem Cell Reports. 2013 Nov 27;1(6):590-603. doi: 10.1016/j.stemcr.2013.10.008. eCollection 2013. Stem Cell Reports. 2013. PMID: 24371812 Free PMC article.
References
- Kingsley PD, Malik J, Fantauzzo KA, Palis J. Yolk sac-derived primitive erythroblasts enucleate during mammalian embryogenesis. Blood. 2004;104(1):19–25. - PubMed
- Muller AM, Medvinsky A, Strouboulis J, Grosveld F, Dzierzak E. Development of hematopoietic stem cell activity in the mouse embryo. Immunity. 1994;1(4):291–301. - PubMed
- Ema H, Nakauchi H. Expansion of hematopoietic stem cells in the developing liver of a mouse embryo. Blood. 2000;95(7):2284–2288. - PubMed
- Kumaravelu P, Hook L, Morrison AM, et al. Quantitative developmental anatomy of definitive haematopoietic stem cells/long-term repopulating units (HSC/RUs): role of the aorta-gonad-mesonephros (AGM) region and the yolk sac in colonisation of the mouse embryonic liver. Development. 2002;129(21):4891–4899. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases