Molecular clustering identifies complement and endothelin induction as early events in a mouse model of glaucoma - PubMed (original) (raw)
. 2011 Apr;121(4):1429-44.
doi: 10.1172/JCI44646. Epub 2011 Mar 7.
Danilo G Macalinao, Gregory L Sousa, Michael Walden, Ileana Soto, Stephen C Kneeland, Jessica M Barbay, Benjamin L King, Jeffrey K Marchant, Matthew Hibbs, Beth Stevens, Ben A Barres, Abbot F Clark, Richard T Libby, Simon W M John
Affiliations
- PMID: 21383504
- PMCID: PMC3069778
- DOI: 10.1172/JCI44646
Molecular clustering identifies complement and endothelin induction as early events in a mouse model of glaucoma
Gareth R Howell et al. J Clin Invest. 2011 Apr.
Abstract
Glaucoma is one of the most common neurodegenerative diseases. Despite this, the earliest stages of this complex disease are still unclear. This study was specifically designed to identify early stages of glaucoma in DBA/2J mice. To do this, we used genome-wide expression profiling of optic nerve head and retina and a series of computational methods. Eyes with no detectable glaucoma by conventional assays were grouped into molecularly defined stages of disease using unbiased hierarchical clustering. These stages represent a temporally ordered sequence of glaucoma states. We then determined networks and biological processes that were altered at these early stages. Early-stage expression changes included upregulation of both the complement cascade and the endothelin system, and so we tested the therapeutic value of separately inhibiting them. Mice with a mutation in complement component 1a (C1qa) were protected from glaucoma. Similarly, inhibition of the endothelin system with bosentan, an endothelin receptor antagonist, was strongly protective against glaucomatous damage. Since endothelin 2 is potently vasoconstrictive and was produced by microglia/macrophages, our data provide what we believe to be a novel link between these cell types and vascular dysfunction in glaucoma. Targeting early molecular events, such as complement and endothelin induction, may provide effective new treatments for human glaucoma.
Figures
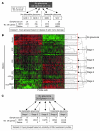
Figure 1. Early stages of glaucoma identified by hierarchical clustering.
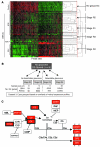
(A) Pairwise comparisons for eyes grouped by conventional morphological assessment of optic nerve damage (Dataset 1). The numbers of DE genes compared with controls are shown for both the ONH and retina. NOE eyes had no detectable glaucoma; MOD and SEV eyes had readily detectable damage and significant axon loss (Supplemental Figure 1). A total of 40 DBA/2J eyes were compared with the 10 controls. (B) A glaucoma-relevant probe set (see Methods and Supplemental Figure 1) was used to cluster the same eyes into molecular stages based on expression profiles of ONH samples. The expression data for all 50 eyes are shown. Five molecularly defined stages containing at least 4 eyes were identified (ONH stages 1–5). The optic nerve damage level is indicated on the left for control, NOE, and SEV eyes. All unlabeled eyes had MOD glaucoma. Black horizontal lines indicate borders between stages. The red line indicates the cutoff level for inclusion in a stage. Raw normalized intensity values are represented as green to black to red. For each probe set, green represents the lower normalized intensity values across all eyes and red the higher intensity values. All normalized intensity values for each probe set are available in Geo DataSets (GSE26299). (C) Pairwise comparisons for these molecular stages (Dataset 2). Stage 3 contains 3 eyes with no detectable glaucoma and 3 eyes with detectable glaucoma. The numbers of DE genes for the ONH and retina for each stage are shown.
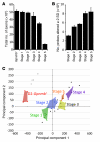
Figure 2. Differences between the molecularly defined ONH stages.
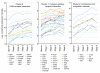
(A) Overall, axon number decreased, from stage 1 to 5. However, for stages 1–3, there was no significant axon loss with respect to D2-Gpnmb+ control eyes (P > 0.1). (B) The number of probe sets altered by at least 2 SDs (with respect to D2-Gpnmb+ control) increased with disease stage. (C) PCA showed that stages 1–5 occupy essentially non-overlapping territories. D2-Gpnmb+ control samples that did not cluster (open diamonds, upper left quadrant) were clearly distinct from DBA/2J glaucoma samples (filled colored diamonds). DBA/2J samples that did not cluster are shown as filled black diamonds.
Figure 3. Clustered stages allow sensitive detection of early changes in the ONH.
(A) The number of DE genes in morphologically defined dataset 1 was compared with the number of DE genes in molecularly defined dataset 2. 1,385 genes were DE at least 2-fold compared with controls in molecularly defined stages 1–3. This is in comparison to only 100 genes for the NOE2 group. Importantly, the expression patterns that define stages 1–3 represent changes that occur prior to detectable morphological damage. (B) The fold changes for individual genes were often 2 or 3 times greater for the molecularly compared with morphologically defined groups. For this figure, stage 3 is included to the left of the line indicating detectable glaucoma. This is because the gene expression differences that determine inclusion in this stage occur prior to detectable optic nerve damage. (C) The molecularly defined stages allow identification of more DE genes with a particular GO term than the morphologically defined data. The expression of genes with immune-modulating functions, including chemotaxis and leukocyte activation, change early in the ONH. (D) KEGG pathway analysis suggests that ECM-receptor interactions, MAPK signaling, and Toll-like receptor signaling pathways are among the earliest pathways to become activated. *q ≤ 0.05 compared with Gpnmb+ controls.
Figure 4. Genes in the ECM-receptor interaction pathway are upregulated in the ONH.
(A) DE genes in the ECM-receptor interaction pathway that are upregulated in stage 2 are shown in red. These genes remain upregulated through stage 5. Reproduced with permission from KEGG (
http://www.genome.jp/kegg/kegg1.html
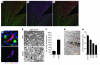
) (B) Fold changes for 5 genes within the ECM pathway. Lamc1, Col5a2, and Spp1 are DE (q ≤ 0.05) across all stages. Itga5 and Tnc are DE across stages 2–5. (C) RNA in situ hybridization for Tnc, the most highly expressed gene in the ECM-receptor interaction pathway. Tnc is largely expressed in the glial lamina (indicated by vertical white lines) of the ONH and is likely present in astrocytes. V, vessel; NFL, neurofilament (detected with an antibody; see Methods). Scale bars: top 2 rows, 50 μm; bottom row, 20 μm.
Figure 5. K-means clustering identified pathways changing in early stages of glaucoma.
K-means clustering was used to partition genes that change in the ONH into clusters (Table 1). Genes within a cluster behaved in a similar fashion as glaucoma progressed. Gene expression patterns in 3 overrepresented pathways are shown. Profiles are based on normalized raw expression values across D2-Gpnmb+ control and glaucoma stages 1–5.
Figure 6. C1qa expression in microglia in the ONH.
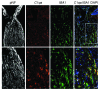
C1qa expression (red, riboprobe) occurs in microglia (IBA1 marker, green, antibody) in the ONH during DBA/2J glaucoma. All panels show the same eye, which had no detectable optic nerve damage (NOE). The boxed area is enlarged in the lower panels. Phosphorylated neurofilament (pNF) labels healthy axons throughout the ONH. DAPI labels nuclei. Scale bars: top panels, 50 μm; bottom panels, 20 μm. Supplemental Figure 3 shows other complement genes that are DE in the ONH.
Figure 7. Molecular clustering identifies early stages of glaucoma in the retina.
(A) 600 glaucoma-relevant probe sets that were DE in retina were used to cluster samples into molecularly defined stages (retinal stages R1–R4). 48 of the 50 eyes in the study were clustered (quality control failed for 2 retinal microarrays; see Methods). Expression data for all 48 eyes are shown (normalized intensity values have been submitted to GEO). Although eyes are grouped based on the similarity of gene expression, the damage level based on morphological analysis is indicated on the left for control, NOE, and SEV eyes. Unlabeled eyes had MOD glaucoma. Black horizontal lines indicate borders between stages. The red line indicates the cutoff level for inclusion in a stage. (B) Pairwise comparisons for the new molecularly defined retinal stages. The number of DE genes for ONH and retina for each stage are shown. Importantly, stages R1 and R2 were previously indistinguishable, with no detectable glaucomatous optic nerve damage. Compared with the other stages, stage R3 lacks power, as it contains 3 eyes. (C) Pathway analysis (using DAVID, see Methods) of the DE genes in stage R1 and R2 (compared with control) identified the complement cascade as the most significantly overenriched pathway (KEGG mmu04610). Genes shown in red have greater expression in molecularly defined retinal stage R2 than Gpnmb+ controls. Three of these genes were DE in stage R1 (surrounded by thick black boxes).
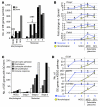
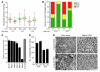
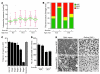
Figure 8. C1QA deficiency protects against DBA/2J glaucoma.
(A) Despite a delay in IOP elevation in some eyes, IOPs of C1qa WT and homozygous mutant (KO) mice overlap extensively (WT versus KO: 8.5 months, P = 0.026; 10.5 months, P = 0.34). Number of assessed eyes was at least 43 for all groups (B) The vast majority of optic nerves from C1qa mutant mice are indistinguishable from control nerves with no detectable glaucoma (NOE, green bars) at 10.5 months of age, and retain a lower glaucoma incidence than WT mice at 12 months of age. Sample sizes: 46 WT, 45 KO at 10.5 months; 62 WT, 56 KO at 12 months. P < 1 × 10–8 at each age comparing KO with WT distributions. (C) C1qa KO eyes with NOE nerve damage (n = 10) have axon number similar to that of no-glaucoma Gpnmb+ controls (n = 11, P = 0.26). For ease of comparison, axon counts for stages 1–5 are repeated from Figure 1. (D) In the ganglion cell layer (GCL), RGC somata are also spared in C1qa mutants with NOE nerve damage, but the number of RGC soma lost in KO eyes with severe optic nerve damage was similar to that in WT counterparts. Amacrine cells make up the majority of remaining GCL cells in severely affected eyes. (E) The most common optic nerve and GCL phenotypes for 12-month-old mice of each genotype are shown. Optic nerve cross sections were stained with PPD and flat mounted retinas stained with cresyl violet. Scale bar: 50 μm.
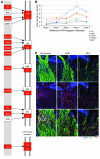
Figure 9. EDN2 is expressed in retinal microglia and may mediate early vascular damage in glaucoma.
(A–C) Images of a retina from a glaucomatous DBA/2J eye with fluorescently marked RGCs (D2._Thy1_-CFP mice; RGCs and their axons are green). EDN2 (magenta) is present in IBA1-positive cells (red) in the RGC and nerve fiber layers. IBA1 is a marker of microglia and macrophages. (D) High-resolution images of an EDN2-positive microglia (IBA1, upper panel; EDN2, lower panel). (E–F) Intravitreal injection of EDN2 peptide causes RGC axon damage. EDN2 peptide (500 μm) or vehicle (PBS) was injected into the vitreous and RGC axon damage assessed 4 weeks later. There was a significant increase in the level of RGC axon damage in EDN2-injected eyes compared with PBS-treated control eyes (n = 5 control, n = 8 EDN2 eyes, P = 0.0074). (G) To assess vascular changes, retinas were visualized by DIC, and the ratio of lumen area (calculated from the lumen diameter, yellow arrows; see Methods) to total vessel area (calculated from vessel diameter, red arrows) was measured. (H) There is a significant decrease in the lumen/vessel ratio in eyes with increasing levels of glaucoma (P < 0.001). Scale bars: A–C, 100 μm, D, 10 μm, E, 50 μm.
Figure 10. Bosentan robustly protects from glaucoma in DBA/2J glaucoma.
(A) Administration of bosentan (an endothelin receptor antagonist) does not alter IOP elevation in DBA/2J mice. Sample size: 39–47 per group. (B) Bosentan administration protects significant numbers of eyes from glaucoma (10.5 months, P = 1 × 10–26; 12 months, P = 0.01; sample sizes: 54 untreated, 54 treated at 10.5 months and 58 untreated, 58 treated at 12 months). (C) Randomly selected mice that were treated with bosentan and had the NOE nerve damage level have an axon number similar to that in no-glaucoma Gpnmb+ controls (11 treated vs. 13 Gpnmb+, P = 0.5) and in DBA/2J mice prior to glaucomatous axon loss. For ease of comparison, axon counts for stages 1–5 are repeated from Figure 1. (D) There is no significant soma loss in bosentan-protected eyes (NOE). Not unexpectedly, Bosentan did not save RGC soma in eyes with severe axon loss (SEV). (E) Representative optic nerve and retinal phenotypes for mice of each treatment group are shown. Optic nerve cross sections were stained with PPD; retinas with cresyl violet. Scale bar: 50 μm.
Similar articles
- Combinatorial targeting of early pathways profoundly inhibits neurodegeneration in a mouse model of glaucoma.
Howell GR, MacNicoll KH, Braine CE, Soto I, Macalinao DG, Sousa GL, John SW. Howell GR, et al. Neurobiol Dis. 2014 Nov;71:44-52. doi: 10.1016/j.nbd.2014.07.016. Epub 2014 Aug 15. Neurobiol Dis. 2014. PMID: 25132557 Free PMC article. - Early immune responses are independent of RGC dysfunction in glaucoma with complement component C3 being protective.
Harder JM, Braine CE, Williams PA, Zhu X, MacNicoll KH, Sousa GL, Buchanan RA, Smith RS, Libby RT, Howell GR, John SWM. Harder JM, et al. Proc Natl Acad Sci U S A. 2017 May 9;114(19):E3839-E3848. doi: 10.1073/pnas.1608769114. Epub 2017 Apr 26. Proc Natl Acad Sci U S A. 2017. PMID: 28446616 Free PMC article. - Complement component 1Q (C1Q) upregulation in retina of murine, primate, and human glaucomatous eyes.
Stasi K, Nagel D, Yang X, Wang RF, Ren L, Podos SM, Mittag T, Danias J. Stasi K, et al. Invest Ophthalmol Vis Sci. 2006 Mar;47(3):1024-9. doi: 10.1167/iovs.05-0830. Invest Ophthalmol Vis Sci. 2006. PMID: 16505037 - A role for complement in glaucoma?
Ren L, Danias J. Ren L, et al. Adv Exp Med Biol. 2010;703:95-104. doi: 10.1007/978-1-4419-5635-4_7. Adv Exp Med Biol. 2010. PMID: 20711709 Review. - [Aiming for zero blindness].
Nakazawa T. Nakazawa T. Nippon Ganka Gakkai Zasshi. 2015 Mar;119(3):168-93; discussion 194. Nippon Ganka Gakkai Zasshi. 2015. PMID: 25854109 Review. Japanese.
Cited by
- Isolation of intact astrocytes from the optic nerve head of adult mice.
Choi HJ, Sun D, Jakobs TC. Choi HJ, et al. Exp Eye Res. 2015 Aug;137:103-10. doi: 10.1016/j.exer.2015.06.014. Epub 2015 Jun 17. Exp Eye Res. 2015. PMID: 26093274 Free PMC article. - Rod photoreceptor ribbon synapses in DBA/2J mice show progressive age-related structural changes.
Fuchs M, Scholz M, Sendelbeck A, Atorf J, Schlegel C, Enz R, Brandstätter JH. Fuchs M, et al. PLoS One. 2012;7(9):e44645. doi: 10.1371/journal.pone.0044645. Epub 2012 Sep 5. PLoS One. 2012. PMID: 22957094 Free PMC article. - Elevation of intraocular pressure in rodents using viral vectors targeting the trabecular meshwork.
Pang IH, Millar JC, Clark AF. Pang IH, et al. Exp Eye Res. 2015 Dec;141:33-41. doi: 10.1016/j.exer.2015.04.003. Epub 2015 May 27. Exp Eye Res. 2015. PMID: 26025608 Free PMC article. Review. - Neurodegeneration severity can be predicted from early microglia alterations monitored in vivo in a mouse model of chronic glaucoma.
Bosco A, Romero CO, Breen KT, Chagovetz AA, Steele MR, Ambati BK, Vetter ML. Bosco A, et al. Dis Model Mech. 2015 May;8(5):443-55. doi: 10.1242/dmm.018788. Epub 2015 Mar 9. Dis Model Mech. 2015. PMID: 25755083 Free PMC article. - The cell stress machinery and retinal degeneration.
Athanasiou D, Aguilà M, Bevilacqua D, Novoselov SS, Parfitt DA, Cheetham ME. Athanasiou D, et al. FEBS Lett. 2013 Jun 27;587(13):2008-17. doi: 10.1016/j.febslet.2013.05.020. Epub 2013 May 15. FEBS Lett. 2013. PMID: 23684651 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- R01 EY011721/EY/NEI NIH HHS/United States
- R29 EY011721/EY/NEI NIH HHS/United States
- R01 EY018606-04/EY/NEI NIH HHS/United States
- EY018606/EY/NEI NIH HHS/United States
- R01 EY018606/EY/NEI NIH HHS/United States
- F32 EY014515/EY/NEI NIH HHS/United States
- T32 NS51112-05/NS/NINDS NIH HHS/United States
- EY011721/EY/NEI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- T32 NS051112/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous