Expression and cellular localization of cyclooxygenases and prostaglandin E synthases in the hemorrhagic brain - PubMed (original) (raw)
Expression and cellular localization of cyclooxygenases and prostaglandin E synthases in the hemorrhagic brain
Tao Wu et al. J Neuroinflammation. 2011.
Abstract
Background: Although cyclooxygenases (COX) and prostaglandin E synthases (PGES) have been implicated in ischemic stroke injury, little is known about their role in intracerebral hemorrhage (ICH)-induced brain damage. This study examines the expression and cellular localization of COX-1, COX-2, microsomal PGES-1 (mPGES-1), mPGES-2, and cytosolic PGES (cPGES) in mice that have undergone hemorrhagic brain injury.
Methods: ICH was induced in C57BL/6 mice by intrastriatal injection of collagenase. Expression and cellular localization of COX-1, COX-2, mPGES-1, mPGES-2, and cPGES were examined by immunofluorescence staining.
Results: In the hemorrhagic brain, COX-1, mPGES-2, and cPGES were expressed constitutively in neurons; COX-1 was also constitutively expressed in microglia. The immunoreactivity of COX-2 was increased in neurons and astrocytes surrounding blood vessels at 5 h and then tended to decrease in neurons and increase in astrocytes at 1 day. At 3 days after ICH, COX-2 was observed primarily in astrocytes but was absent in neurons. Interestingly, the immunoreactivity of mPGES-1 was increased in neurons in the ipsilateral cortex and astrocytes in the ipsilateral striatum at 1 day post-ICH; the immunoreactivity of astrocytic mPGES-1 further increased at 3 days.
Conclusion: Our data suggest that microglial COX-1, neuronal COX-2, and astrocytic COX-2 and mPGES-1 may work sequentially to affect ICH outcomes. These findings have implications for efforts to develop anti-inflammatory strategies that target COX/PGES pathways to reduce ICH-induced secondary brain damage.
Figures
Figure 1
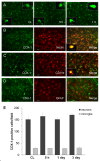
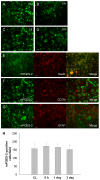
Immunostaining of COX-1 and cell type-specific proteins in the mouse brain after ICH. A: COX-1 was constitutively expressed in the contralateral striatum (CL) and did not change significantly from 5 h to 1 day after ICH. The insets in A are representative COX-1-expressing cells at higher magnification (Scale bar, 10 μm). B-D: Double immunostaining of COX-1 with cell type-specific markers for neurons (NeuN), microglia (CD11b), and astrocytes (GFAP) in the striatum surrounding the hematoma at 3 days after ICH. Insets in B and C show a double-stained cell at higher magnification (Scale bar, 10 μm). Scale bar in the middle panels of B, C, D, 30 μm. E: Quantification analysis confirmed that the number of COX-1-immunoreactive neurons and microglia did not change in the perihematomal region of the striatum from 5 h to 3 days after ICH (n = 3/group, all P > 0.05). Values are means ± S.D.
Figure 2
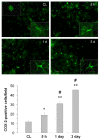
Immunostaining of COX-2 in the mouse brain after ICH. Minimal COX-2 immunoreactivity was observed in neuron-like and glia-like cells in the contralateral striatum (CL). After ICH, the number of strongly COX-2-immunoreactive cells increased in the perihematomal region of the striatum; the increase started at 5 h and continued at 3 days. Scale bar, 30 μm. Insets are representative COX-2-expressing cells at higher magnification (Scale bar, 15 μm). Quantification analysis confirmed that the number of strongly COX-2-immunoreactive cells increased from 5 h to 3 days in the perihematomal region of the striatum (n = 3/group, * P < 0.05, ** P < 0.01 compared with the CL, # P < 0.01 compared with the previous time point). Values are means ± S.D.
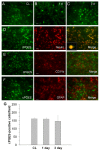
Figure 3
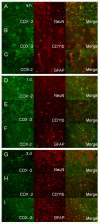
Double immunostaining of COX-2 with cell type-specific markers (NeuN for neurons, CD11b for microglia, and GFAP for astrocytes) in the mouse brain after ICH. A-C: In the perihematomal region of the striatum, the number of COX-2-immunoreactive cells increased; double immunostaining demonstrated that COX-2 was expressed in neurons (A) and astrocytes (C), but not in microglia (B), at 5 h after ICH. D-F: At 1 day after ICH, the COX-2 immunoreactivity tended to decrease in neurons (D) and increase in astrocytes (F) of the perihematomal region in the striatum; however COX-2 immunoreactivity remained unchanged in microglia (E). G-I: At 3 days post-ICH, COX-2 immunoreactivity further increased in astrocytes (I) but was not observed in neurons (G) or microglia (H) in the perihematomal region of the striatum. Scale bar in C, F, I, 30 μm.
Figure 4
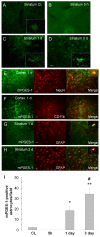
Immunostaining of mPGES-1 and cell type-specific proteins (NeuN for neurons, CD11b for microglia, and GFAP for astrocytes) in the mouse brain after ICH. (A) Minimal immunoreactivity of mPGES-1 was detected in neuron-like cells in the contralateral striatum (CL). B-D: In the perihematomal region of the striatum, mPGES-1 immunoreactivity was undetectable at 5 h (B) but began to increase in glia-like cells at 1 day (C). It was additionally increased in glia-like cells at 3 days (D). Insets in A, C, and D are representative mPGES-1-expressing cells at higher magnification (Scale bar, 15 μm). E-F: Double immunostaining showed induction of mPGES-1 in neurons (E), but not in microglia (F), in the ipsilateral frontal-parietal cortex at 1 day. G-H: In the perihematomal region of the striatum, double immunostaining showed that mPGES-1 was induced primarily in astrocytes at 1 day (G); the number of mPGES-1-immunoreactive astrocytes was additionally increased at 3 days (H). Scale bar in D-H, 30 μm. Insets in E, G, and H are double-stained cells at higher magnification (Scale bar, 15 μm). Quantification analysis (I) confirmed that the number of mPGES-1 immunoreactive cells increased at 1 day, and further increased at 3 days in the perihematomal region of the striatum (n = 3/group, * P < 0.05, ** P < 0.01 compared with the CL, # P < 0.01 compared with day 1). Values are means ± S.D.
Figure 5
Immunostaining of mPGES-2 and cell type-specific proteins (NeuN for neurons, CD11b for microglia, and GFAP for astrocytes) in the mouse brain after ICH. A: Immunoreactive staining for mPGES-2 was observed in neuron-like cells in the contralateral striatum (CL). B-D: In the perihematomal region of the striatum, mPGES-2 immunoreactivity did not change significantly between 5 h (B) and 1 day (C) or 3 days (D) post-ICH. E-G: In the perihematomal region of the striatum, double immunostaining revealed that mPGES-2 was present in neurons at 1 day (E), but not in microglia at 1 day (F) or astrocytes at 3 days (G). Scale bar in D-G, 30 μm. Inset in E is a double-stained cell at higher magnification (Scale bar, 20 μm). Quantification analysis (H) confirmed that the number of mPGES-2-immunoreactive cells did not change in the perihematomal region of the striatum from 5 h to 3 days after ICH (n = 3/group, all P > 0.05). Values are means ± S.D.
Figure 6
Immunostaining of cPGES and cell type-specific proteins (NeuN for neurons, CD11b for microglia, and GFAP for astrocytes) in the mouse brain after ICH. A: Immunoreactive staining for cPGES was observed in neuron-like cells in the contralateral striatum (CL). B-C: In the perihematomal region of the striatum, cPGES immunoreactivity did not change significantly between 1 day (B) and 3 days (C). D-F: In the perihematomal region of the striatum, double immunostaining showed that cPGES was present in neurons (D), but not in microglia (E) or astrocytes (F), at 1 day. Scale bar in B-F, 30 μm. Inset in D is a double-stained cell at higher magnification (Scale bar, 15 μm). Quantification analysis (G) confirmed that the number of cPGES immunoreactive cells did not change in the perihematomal region of the striatum between 1 day and 3 days after ICH (n = 3/group, all P > 0.05). Values are means ± S.D.
Similar articles
- Microsomal prostaglandin E synthase (mPGES)-1, mPGES-2 and cytosolic PGES expression in human gastritis and gastric ulcer tissue.
Gudis K, Tatsuguchi A, Wada K, Futagami S, Nagata K, Hiratsuka T, Shinji Y, Miyake K, Tsukui T, Fukuda Y, Sakamoto C. Gudis K, et al. Lab Invest. 2005 Feb;85(2):225-36. doi: 10.1038/labinvest.3700200. Lab Invest. 2005. PMID: 15531909 - Intracellular-specific colocalization of prostaglandin E2 synthases and cyclooxygenases in the brain.
Vazquez-Tello A, Fan L, Hou X, Joyal JS, Mancini JA, Quiniou C, Clyman RI, Gobeil F Jr, Varma DR, Chemtob S. Vazquez-Tello A, et al. Am J Physiol Regul Integr Comp Physiol. 2004 Nov;287(5):R1155-63. doi: 10.1152/ajpregu.00077.2004. Epub 2004 Jul 29. Am J Physiol Regul Integr Comp Physiol. 2004. PMID: 15284079 - Microglia-specific expression of microsomal prostaglandin E2 synthase-1 contributes to lipopolysaccharide-induced prostaglandin E2 production.
Ikeda-Matsuo Y, Ikegaya Y, Matsuki N, Uematsu S, Akira S, Sasaki Y. Ikeda-Matsuo Y, et al. J Neurochem. 2005 Sep;94(6):1546-58. doi: 10.1111/j.1471-4159.2005.03302.x. Epub 2005 Jul 5. J Neurochem. 2005. PMID: 16000148 - Membrane prostaglandin E synthase-1: a novel therapeutic target.
Samuelsson B, Morgenstern R, Jakobsson PJ. Samuelsson B, et al. Pharmacol Rev. 2007 Sep;59(3):207-24. doi: 10.1124/pr.59.3.1. Pharmacol Rev. 2007. PMID: 17878511 Review. - Prostaglandin E synthase.
Murakami M, Nakatani Y, Tanioka T, Kudo I. Murakami M, et al. Prostaglandins Other Lipid Mediat. 2002 Aug;68-69:383-99. doi: 10.1016/s0090-6980(02)00043-6. Prostaglandins Other Lipid Mediat. 2002. PMID: 12432931 Review.
Cited by
- Autophagy upregulation and apoptosis downregulation in DAHP and triptolide treated cerebral ischemia.
Yang Y, Gao K, Hu Z, Li W, Davies H, Ling S, Rudd JA, Fang M. Yang Y, et al. Mediators Inflamm. 2015;2015:120198. doi: 10.1155/2015/120198. Epub 2015 Feb 2. Mediators Inflamm. 2015. PMID: 25729215 Free PMC article. - Diet-Induced High Serum Levels of Trimethylamine-N-oxide Enhance the Cellular Inflammatory Response without Exacerbating Acute Intracerebral Hemorrhage Injury in Mice.
Li C, Zhu L, Dai Y, Zhang Z, Huang L, Wang TJ, Fu P, Li Y, Wang J, Jiang C. Li C, et al. Oxid Med Cell Longev. 2022 Feb 16;2022:1599747. doi: 10.1155/2022/1599747. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35242275 Free PMC article. - PGE2-EP3 signaling exacerbates intracerebral hemorrhage outcomes in 24-mo-old mice.
Leclerc JL, Lampert AS, Diller MA, Doré S. Leclerc JL, et al. Am J Physiol Heart Circ Physiol. 2016 Jun 1;310(11):H1725-34. doi: 10.1152/ajpheart.00638.2015. Epub 2016 Apr 15. Am J Physiol Heart Circ Physiol. 2016. PMID: 27084388 Free PMC article. - Delayed administration of parecoxib, a specific COX-2 inhibitor, attenuated postischemic neuronal apoptosis by phosphorylation Akt and GSK-3β.
Ye Z, Wang N, Xia P, Wang E, Yuan Y, Guo Q. Ye Z, et al. Neurochem Res. 2012 Feb;37(2):321-9. doi: 10.1007/s11064-011-0615-y. Epub 2011 Sep 30. Neurochem Res. 2012. PMID: 21964800 - Progesterone Changes VEGF and BDNF Expression and Promotes Neurogenesis After Ischemic Stroke.
Jiang C, Zuo F, Wang Y, Lu H, Yang Q, Wang J. Jiang C, et al. Mol Neurobiol. 2016 Jan 8:10.1007/s12035-015-9651-y. doi: 10.1007/s12035-015-9651-y. Online ahead of print. Mol Neurobiol. 2016. PMID: 26746666 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous