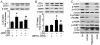2-Deoxy-D-glucose treatment of endothelial cells induces autophagy by reactive oxygen species-mediated activation of the AMP-activated protein kinase - PubMed (original) (raw)
2-Deoxy-D-glucose treatment of endothelial cells induces autophagy by reactive oxygen species-mediated activation of the AMP-activated protein kinase
Qilong Wang et al. PLoS One. 2011.
Abstract
Autophagy is a cellular self-digestion process activated in response to stresses such as energy deprivation and oxidative stress. However, the mechanisms by which energy deprivation and oxidative stress trigger autophagy remain undefined. Here, we report that activation of AMP-activated protein kinase (AMPK) by mitochondria-derived reactive oxygen species (ROS) is required for autophagy in cultured endothelial cells. AMPK activity, ROS levels, and the markers of autophagy were monitored in confluent bovine aortic endothelial cells (BAEC) treated with the glycolysis blocker 2-deoxy-D-glucose (2-DG). Treatment of BAEC with 2-DG (5 mM) for 24 hours or with low concentrations of H(2)O(2) (100 µM) induced autophagy, including increased conversion of microtubule-associated protein light chain 3 (LC3)-I to LC3-II, accumulation of GFP-tagged LC3 positive intracellular vacuoles, and increased fusion of autophagosomes with lysosomes. 2-DG-treatment also induced AMPK phosphorylation, which was blocked by either co-administration of two potent anti-oxidants (Tempol and N-Acetyl-L-cysteine) or overexpression of superoxide dismutase 1 or catalase in BAEC. Further, 2-DG-induced autophagy in BAEC was blocked by overexpressing catalase or siRNA-mediated knockdown of AMPK. Finally, pretreatment of BAEC with 2-DG increased endothelial cell viability after exposure to hypoxic stress. Thus, AMPK is required for ROS-triggered autophagy in endothelial cells, which increases endothelial cell survival in response to cell stress.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
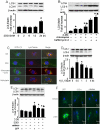
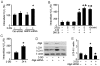
Figure 1. 2-DG-induced autophagy is ROS-dependent.
A: Confluent monolayers of BAEC were treated with 5 mM 2-DG for the indicated times. Cell lysates were analyzed by Western blot using antibody against LC3B. (n = 3; one-way ANOVA: *p<0.05 vs control). B: Confluent monolayers of BAEC were treated with 5 mM 2-DG for 24 hrs in the presence or absence of chloroquine (3 µM) or bafilomycin A (10 nM). Cell lysates were analyzed by Western blot for detection of LC3. (n = 3; two-way ANOVA: *p<0.05 2-DG vs vehicle, 2-DG + chloroquine vs. chloroquine, 2-DG + bafilomycin A vs. bafilomycin A. p<0.05, 2-DG vs. 2-DG + bafilomycin A or 2-DG + chloroquine). C: BAEC expressing GFP-LC3 were treated with chloroquine or 2-DG, and the accumulation of LC3 II (green), localization of LC3 II with lysosomes (red), and DAPI (blue) staining of nuclei in response to treatment were analyzed by fluorescence microscopy. Scale bars, 5 µm. D: Confluent monolayers of BAEC were treated with 100 µM H2O2 for the indicated times. Cell lysates analyzed by Western blot using antibody against LC3 (n = 3; one-way ANOVA: *p<0.05 vs control). E: BAEC were transduced adenovirus vectors encoding SOD1 or catalase for 48 hrs and then exposed to 5 mM 2-DG for 24 hrs (n = 3; two-way ANOVA, * p<0.05, GFP vs. 2-DG, SOD1 vs 2-DG + SOD1, catalase vs 2-DG + catalase, p<0.05, 2-DG vs. 2-DG + catalase overexpression). F: The accumulation of LC3 II (green), localization of LC3 II lysosomes (red), and DAPI (blue) staining of nuclei in response to SOD1 or catalase overexpression and 2-DG treatment were analyzed by fluorescence microscopy.
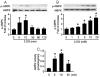
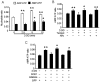
Figure 2. Time-course and dose-response for 2-DG-induced AMPK activation.
A: Confluent BAEC monolayers were treated with 2-DG (5 mM) for the indicated times. Cell lysates were analyzed by Western blot using antibody against p-AMPK and AMPKα1/2 (n = 3; one-way ANOVA: *p<0.05 vs. control). B: Confluent BAEC monolayers were treated with the indicated concentration of 2-DG for 10 min (n = 3; one-way ANOVA: *p<0.05 vs. control). C: BAEC were incubated with 5 mM of 2-DG for the indicated times. AMPK was immunoprecipitated from cell lysis with an antibody against AMPKα bound to Protein A/G agarose overnight at 4°C. AMPK activity was determined by SAMS phosphorylation using a [32P]ATP assay (n = 3; one-way ANOVA, *p<0.05 vs. control).
Figure 3. 2-DG-induced autophagy is AMPK-dependent.
A: BAEC were transduced with adenovirus vectors encoding dominant negative AMPK (AMPK-DN) for 48 hrs and then treated with 5 mM 2-DG for 24 hrs (n = 3; two-way ANOVA, * p<0.05, GFP vs. 2-DG, p<0.05, 2-DG vs. 2-DG + AMPK-DN). B: HUVEC were transfected with AMPK-targeted siRNA or control siRNA for 24 hrs then treated with 5 mM 2-DG for 24 hrs. Cell lysates were analyzed by Western blot using antibody against LC3 (n = 3; two-way ANOVA, * p<0.05, vehicle vs. 2-DG, p<0.05, Control siRNA vs. AMPK siRNA). C: BAEC were treated with 1 mM AICAR, 10 nM rapamycin, or 5 mM 2-DG for 24 hrs and then analyzed by Western blot to determine the level of phospho-AMPK-Thr172 (p-AMPK), phos-ACC-Ser79 (p-ACC), phos-mTOR-Ser2448 (p-mTOR), mTOR, phos-p70S6K-Thr389 (p-p70S6K), p70S6K, phos-4EBP1-Thr37/46 (p-4EBP1), and LC3.
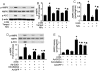
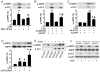
Figure 4. Inhibitory effects of antioxidants on 2-DG-induced AMPK activation in BAEC.
A, B: Confluent BAEC monolayers were pretreated with 10 µM 4-hydroxy-Tempol (Tempol) or 2 mM N-Acetyl-Cysteine (NAc) for 30 min and then treated with 5 mM 2-DG for 10 min. Cell lysates were subjected to Western blot analysis using antibodies against p-AMPK, p-ACC, AMPKα1/2, and β-actin (n = 3; two-way ANOVA,* p<0.05, 2-DG vs. control, Tempol + 2-DG vs. Tempol, NAc + 2-DG vs. Nac, p<0.05 2-DG vs 2-DG + Tem or NAc). C: BAEC were pretreated with NAc and then stimulated with 2-DG. AMPK activity was determined by SAMS phosphorylation using a [32P]ATP assay (n = 3; two-way ANOVA, * p<0.05, vehicle vs 2-DG, Nac vs 2-DG + NAc, p<0.05 2-DG vs. 2-DG + NAc). D, E: Confluent BAEC monolayers were transduced with adenovirus vectors encoding SOD1 or catalase for 48 hrs and then treated with 5 mM 2-DG for 10 min. Phosphorylation of AMPK and AMPK activity were detected, as described in the Matierals and Methods (n = 3; two-way ANOVA, * p<0.05, GFP vs. 2-DG, SOD1 vs 2-DG + SOD1, catalase vs 2-DG + catalase, p<0.05, 2-DG vs. 2-DG + SOD1 or catalase overexpression).
Figure 5. 2-DG increases intracellular synthesis of H2O2 to induce autophagy.
A: HUVEC were transfected with AMPK-targeted siRNA for 24 hrs. Then the cells were incubated in EBM media without phenol red and treated with 5 mM of 2-DG for 10 min. CM-H2DCFDA (10 µM; Invitrogen) was added for 30 min before quantification of fluorescence (excitation at 485 nm and emission at 545 nm). (n = 3; two-way ANOVA,* p<0.05, 2-DG vs. vehicle). B: BAEC were pre-treated with 3-amino-1,2,3-triazine (ATZ, 100 µM) and 1-chloro-2,4-dinitrobenzene (DNCB, 100 µM) for 30 min and then treated with 2-DG for 5 or 10 min. Intracellular H2O2 levels were detected by fluorescence using CM-H2DCFDA. (n = 3; two-way ANOVA,* p<0.05, ATZ + DNCB vs. vehicle, p<0.05, 2-DG + ATZ + DNCB vs. ATZ + DNCB). C: BAEC were treated with 2-DG for 24 hrs. Intracellular H2O2 levels were detected by fluorescence using CM-H2DCFDA. (n = 3; t-test,* p<0.05, 2-DG vs. vehicle). D,E: HUVEC were transfected with Atg4-targeted siRNA or control siRNA for 24 hrs and then treated with 5 mM 2-DG for 24 hrs. Cell lysates were analyzed by Western blot using antibody against LC3 (n = 3; two-way ANOVA, * p<0.05, vehicle vs. 2-DG, p<0.05, Control siRNA vs. Atg4 siRNA).
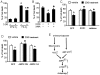
Figure 6. Inhibition of AMPK by antioxidants is independent of the AMP:ATP ratio.
A: Confluent BAEC monolayers were treated with 5 mM of 2-DG for the indicated times. Cells were lysed by perchloric acid and centrifuged as described in the Materials and Methods. The ultrafiltrate was injected into a Jasco HPLC, and ATP, ADP, and AMP were monitored at 260 nm. (n = 4; one-way ANOVA, * p<0.05, ** p<0.01, Control vs. 2-DG treatment). B: BAEC monolayers were pre-treated with 10 µM Tempol or 2 mM NAC and then treated with 5 mM of 2-DG for 5 min. The AMP:ATP ratio in cell lysates was measured by HPLC (n = 5; two-way ANOVA,* p<0.05, ** p<0.01, 2-DG vs. vehicle, Tempol vs. 2-DG + Tempol, NAc vs. 2-DG + NAc). C: BAEC overexpressing SOD1 or catalase were treated with 5 mM 2-DG for 5 min. The AMP:ATP ratio in cell lysates was measured by HPLC. (n = 3; * p<0.05, ** p<0.01, two-way ANOVA, GFP vs. 2-DG, SOD1 vs. 2-DG + SOD1, catalase vs. 2-DG + catalase).
Figure 7. Activation of AMPK by 2-DG is mediated by mitochondrial ROS independent of NAD(P)H oxidase and xanthine oxidase.
A: Confluent BAEC monolayers were pre-treated with 10 µM mito-Tempol for 30 min and then treated with 5 mM of 2-DG for 10 min. Cell lysates were analyzed by Western blot using antibody against p-AMPK (n = 3; two-way ANOVA, *p<0.05, vehicle vs 2-DG, p<0.05, 2-DG vs. 2-DG + mito-Tem). B, C, E: Confluent BAEC monolayers were transduced with adenovirus vectors encoding MnSOD (B), UCP2 (C), or p47phox and p67phox dominant negative mutants (E) for 48 hrs and then treated with 5 mM 2-DG for 10 min. (n = 3; two-way ANOVA, * p<0.05, GFP vs 2-DG + GFP, MnSOD vs 2-DG + Mn SOD, UCP2 vs 2-DG + UCP2, p<0.05 GFP vs. MnSOD or UCP2). D: HUVEC were transfected with control siRNA or UCP-2-targeted siRNA for 24 hrs. Then the cells were treated with 5 mM of 2-DG for 10 min. (n = 3; two-way ANOVA, *p<0.05, 2-DG vs vehicle, p<0.05 control siRNA vs. UCP2 siRNA). F: Confluent BAEC monolayers were pre-treated with allopurinol (100 µM) or oxypurinol (30 µM) for 30 min and then treated with 5 mM of 2-DG for 10 min.
Figure 8. Inhibitory effect of 2-DG-induced autophagy on cell death during hypoxia is dependent on ROS and AMPK.
A: BAEC cells were incubated in the absence 5 mM 2-DG for 24 hrs under normoxia (21% oxygen) or hypoxia (1% oxygen) conditions for 12 hrs. LDH in media was measured to assess cytotoxicity. (n = 4; two-way ANOVA, * p<0.05, normoxia vs hypoxia, p<0.05 vehicle vs. 2-DG treatment). B: BAEC were pretreated with 3-methyladenine (3-MA) for 0.5 hr and then treated with 5 mM 2-DG for 24 hrs. Then, BAEC were incubated in hypoxic conditions for 12 hrs. (n = 4; * p<0.05, two-way ANOVA, vehicle vs. 2-DG treatment, p<0.05, 2-DG vs. 2-DG + 3-methyladenine). C: BAEC were transduced with adenovirus vectors encoding SOD1 or catalase for 48 hrs and then treated with 5 mM 2-DG for 24 hrs. Then, BAEC were incubated in hypoxic conditions for 12 hrs. (n = 4; two-way ANOVA, *p<0.05, vehicle vs. 2-DG treatment, p<0.05, GFP + 2-DG vs. SOD1 or catalase overexpression + 2-DG). D: BAEC were transduced with adenovirus vectors encoding AMPK-DN or AMPK-CA for 48 hrs and then treated with 5 mM 2-DG for 24 hrs. Then, BAEC were incubated in hypoxic conditions for 12 hrs. (n = 4; two-way ANOVA, *p<0.05, vehicle vs. 2-DG treatment, p<0.05, GFP vs. AMPK-DN). E: The model for 2-DG-induced autophagy in endothelial cells. 2-DG increased H2O2 production by mitochondria and the AMP:ATP ratio in endothelial cells. H2O2 subsequently increased AMPK activation, independent of a change in the AMP:ATP ratio. ROS-dependent activation of AMPK by 2-DG is required for autophagy.
Similar articles
- Redox regulation of the AMP-activated protein kinase.
Han Y, Wang Q, Song P, Zhu Y, Zou MH. Han Y, et al. PLoS One. 2010 Nov 5;5(11):e15420. doi: 10.1371/journal.pone.0015420. PLoS One. 2010. PMID: 21079763 Free PMC article. - Starvation-induced autophagy is regulated by mitochondrial reactive oxygen species leading to AMPK activation.
Li L, Chen Y, Gibson SB. Li L, et al. Cell Signal. 2013 Jan;25(1):50-65. doi: 10.1016/j.cellsig.2012.09.020. Epub 2012 Sep 19. Cell Signal. 2013. PMID: 23000343 - Coordinated activation of AMP-activated protein kinase, extracellular signal-regulated kinase, and autophagy regulates phorbol myristate acetate-induced differentiation of SH-SY5Y neuroblastoma cells.
Zogovic N, Tovilovic-Kovacevic G, Misirkic-Marjanovic M, Vucicevic L, Janjetovic K, Harhaji-Trajkovic L, Trajkovic V. Zogovic N, et al. J Neurochem. 2015 Apr;133(2):223-32. doi: 10.1111/jnc.12980. Epub 2014 Nov 14. J Neurochem. 2015. PMID: 25348263 - Exogenous H2S Inhibits Autophagy in Unilateral Ureteral Obstruction Mouse Renal Tubule Cells by Regulating the ROS-AMPK Signaling Pathway.
Chen Q, Yu S, Zhang K, Zhang Z, Li C, Gao B, Zhang W, Wang Y. Chen Q, et al. Cell Physiol Biochem. 2018;49(6):2200-2213. doi: 10.1159/000493824. Epub 2018 Sep 26. Cell Physiol Biochem. 2018. PMID: 30257249 - Lutein Induces Autophagy via Beclin-1 Upregulation in IEC-6 Rat Intestinal Epithelial Cells.
Chang CJ, Lin JF, Hsiao CY, Chang HH, Li HJ, Chang HH, Lee GA, Hung CF. Chang CJ, et al. Am J Chin Med. 2017;45(6):1273-1291. doi: 10.1142/S0192415X17500707. Am J Chin Med. 2017. PMID: 28893091
Cited by
- Low glucose dependent decrease of apoptosis and induction of autophagy in breast cancer MCF-7 cells.
Krętowski R, Borzym-Kluczyk M, Stypułkowska A, Brańska-Januszewska J, Ostrowska H, Cechowska-Pasko M. Krętowski R, et al. Mol Cell Biochem. 2016 Jun;417(1-2):35-47. doi: 10.1007/s11010-016-2711-4. Epub 2016 May 9. Mol Cell Biochem. 2016. PMID: 27160935 Free PMC article. - Beet root juice protects against doxorubicin toxicity in cardiomyocytes while enhancing apoptosis in breast cancer cells.
Das S, Filippone SM, Williams DS, Das A, Kukreja RC. Das S, et al. Mol Cell Biochem. 2016 Oct;421(1-2):89-101. doi: 10.1007/s11010-016-2789-8. Epub 2016 Aug 26. Mol Cell Biochem. 2016. PMID: 27565811 - Dihydroartemisinin inhibits glucose uptake and cooperates with glycolysis inhibitor to induce apoptosis in non-small cell lung carcinoma cells.
Mi YJ, Geng GJ, Zou ZZ, Gao J, Luo XY, Liu Y, Li N, Li CL, Chen YQ, Yu XY, Jiang J. Mi YJ, et al. PLoS One. 2015 Mar 23;10(3):e0120426. doi: 10.1371/journal.pone.0120426. eCollection 2015. PLoS One. 2015. PMID: 25799586 Free PMC article. - Calcineurin suppresses AMPK-dependent cytoprotective autophagy in cardiomyocytes under oxidative stress.
He H, Liu X, Lv L, Liang H, Leng B, Zhao D, Zhang Y, Du Z, Chen X, Li S, Lu Y, Shan H. He H, et al. Cell Death Dis. 2014 Jan 16;5(1):e997. doi: 10.1038/cddis.2013.533. Cell Death Dis. 2014. PMID: 24434520 Free PMC article. - Glucosamine Extends the Lifespan of Caenorhabditis elegans via Autophagy Induction.
Shintani T, Kosuge Y, Ashida H. Shintani T, et al. J Appl Glycosci (1999). 2018 Aug 20;65(3):37-43. doi: 10.5458/jag.jag.JAG-2018_002. eCollection 2018. J Appl Glycosci (1999). 2018. PMID: 34354511 Free PMC article.
References
- Yoo BH, Wu X, Derouet M, Haniff M, Eskelinen EL, et al. Hypoxia-induced downregulation of autophagy mediator Beclin 1 reduces the susceptibility of malignant intestinal epithelial cells to hypoxia-dependent apoptosis. Autophagy. 2009;5:1166–1179. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL074399/HL/NHLBI NIH HHS/United States
- R01 HL089920/HL/NHLBI NIH HHS/United States
- HL080499/HL/NHLBI NIH HHS/United States
- R01 HL079584/HL/NHLBI NIH HHS/United States
- R01 HL080499/HL/NHLBI NIH HHS/United States
- R01 HL096032/HL/NHLBI NIH HHS/United States
- HL096032/HL/NHLBI NIH HHS/United States
- R01 HL074399/HL/NHLBI NIH HHS/United States
- HL089920/HL/NHLBI NIH HHS/United States
- HL079584/HL/NHLBI NIH HHS/United States
- HL105157/HL/NHLBI NIH HHS/United States
- R01 HL110488/HL/NHLBI NIH HHS/United States
- R01 HL105157/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources