AMPA receptor-induced local brain-derived neurotrophic factor signaling mediates motor recovery after stroke - PubMed (original) (raw)
AMPA receptor-induced local brain-derived neurotrophic factor signaling mediates motor recovery after stroke
Andrew N Clarkson et al. J Neurosci. 2011.
Erratum in
- Correction: Clarkson et al., "AMPA Receptor-Induced Local Brain-Derived Neurotrophic Factor Signaling Mediates Motor Recovery after Stroke".
[No authors listed] [No authors listed] J Neurosci. 2017 Oct 18;37(42):10252. doi: 10.1523/JNEUROSCI.2758-17.2017. J Neurosci. 2017. PMID: 31305597 Free PMC article.
Abstract
Stroke is the leading cause of adult disability. Recovery after stroke shares similar molecular and cellular properties with learning and memory. A main component of learning-induced plasticity involves signaling through AMPA receptors (AMPARs). We systematically tested the role of AMPAR function in motor recovery in a mouse model of focal stroke. AMPAR function controls functional recovery beginning 5 d after the stroke. Positive allosteric modulators of AMPARs enhance recovery of limb control when administered after a delay from the stroke. Conversely, AMPAR antagonists impair motor recovery. The contributions of AMPARs to recovery are mediated by release of brain-derived neurotrophic factor (BDNF) in periinfarct cortex, as blocking local BDNF function in periinfarct cortex blocks AMPAR-mediated recovery and prevents the normal pattern of motor recovery. In contrast to a delayed AMPAR role in motor recovery, early administration of AMPAR agonists after stroke increases stroke damage. These findings indicate that the role of glutamate signaling through the AMPAR changes over time in stroke: early potentiation of AMPAR signaling worsens stroke damage, whereas later potentiation of the same signaling system improves functional recovery.
Figures
Figure 1.
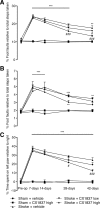
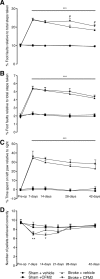
Behavioral recovery in the presence of the high-impact ampakine, CX1837. Behavioral recovery after stroke was assessed on grid-walking (A, B) and cylinder/forelimb asymmetry (C) tasks. Analysis of forelimb (A) and hindlimb (B) footfaults revealed a significant increase in the number of footfaults compared with baseline and time-matched sham-treated controls. Administration of CX1837 (0.33 or 1 mg/kg) resulted in a gradual yet steady dose-dependent decrease in the number of footfaults compared with vehicle (30% HPCD)-treated stroke animals. Assessment of forelimb asymmetry using the cylinder task (C) showed that the mice had a greater tendency to spend more time on their left forepaw poststroke as revealed by an increase in the left/right ratio. Treatment with CX1837 resulted in a steady dose-dependent gain of function of the right forelimb. Data are shown as mean ± SEM for n = 8 per group. **p < 0.01, ***p < 0.001 compared with sham controls; #p < 0.05, ###p < 0.001 compared with stroke plus vehicle-treated animals.
Figure 2.
Behavioral recovery in the presence of the low-impact ampakine, CX1739. Behavioral recovery after stroke was assessed on grid-walking (A, B) and cylinder/forelimb asymmetry (C) tasks. Analysis of forelimb (A) and hindlimb (B) footfaults revealed a significant increase in the number of footfaults compared with baseline and time-matched sham-treated controls. Administration of CX1739 (3 or 30 mg/kg) resulted in a small yet nonsignificant decrease in the number of footfaults compared with vehicle-treated stroke animals. Assessment of forelimb asymmetry using the cylinder task (C) revealed that treatment with CX1739 did not result in a decrease in the left/ratio and were similar to stroke plus vehicle-treated controls. Data are shown as mean ± SEM for n = 8 per group. ***p < 0.001 compared with sham controls.
Figure 3.
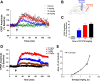
Effects of CX1837 and CX1739 on EPSPs. To assess whether CX1837 and CX1739 crossed the BBB and were having an effect synaptically, EPSPs measures were recorded from anesthetized animals in vivo, with the positioning of the electrode shown in B. Administration of CX1739 (5–20 mg/kg, i.p.) resulted in an immediate increase in EPSP amplitude (A) that was dose dependent (C). CX1837 (0.2–10 mg/kg, i.p.) also resulted in an immediate and dose-dependent increase in EPSP amplitude (D) that is larger in effect than CX1739. The effect of CX1837 is also dose dependent (E). Data points that are shown represent the mean ± SEM. N = 4 per group. **p < 0.01 compared with controls, after analysis using a one-way ANOVA and Dunnett's multiple-comparison test.
Figure 4.
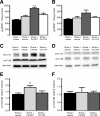
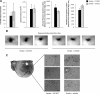
Ampakine-mediated alterations in BDNF expression. CX1837 mediates BDNF release within the periinfarct cortex poststroke. BDNF expression levels (A) were elevated 7 d after stroke. Treatment with CX1837 from day 5 after stroke resulted in a significant increase in BDNF levels, whereas CX1739 did not alter the level of BDNF expression compared with stroke control. No significant changes in BDNF levels were observed on the contralateral hemisphere (B). Assessment of BDNF receptor activation TrkB/p-Trk showed a significant increase in activation after CX1837 treatment within the periinfarct cortex poststroke (C, E). Assessment of TrkB/p-Trk in the contralateral hemisphere showed no changes between treatment groups (D, F). Data are shown as mean ± SEM for n = 4 per group. *p < 0.05, **p < 0.01, ***p < 0.001 compared with sham controls.
Figure 5.
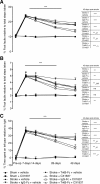
The BDNF ligand decoy, TrkB-Fc, negates the CX1837-mediated gain of behavioral function. BDNF blockade within the periinfarct cortex was achieved by infusing TrkB-Fc-impregnated hydrogel into the stroke cavity. Behavioral recovery was assessed after CX1837 treatment in the presence and absence of TrkB-Fc on grid-walking (A, B) and cylinder/forelimb asymmetry (C) tasks. Implantation of the TrkB-Fc-impregnated hydrogel on day 5 after stroke resulted in a complete blockade of the CX1837-mediated gain of behavioral function on both the grid-walking and cylinder task. Furthermore, vehicle-treated stroke animals that received the TrkB-Fc hydrogel showed impairment in the normal gain of behavioral recovery for hindlimb footfaults (B). These results show a requirement for local periinfarct BDNF levels in facilitating functional recovery. The tables next to A–C show the statistical comparisons between treatment groups at 42 d after stroke. Data are shown as mean ± SEM for n = 8 per group. ns, No significance. **p < 0.01, ***_p_ < 0.001 compared with sham controls; ##_p_ < 0.01, #_p_ < 0.001 compared with stroke plus vehicle-treated animals; >p < 0.001 compared with stroke plus CX1837-treated animals.
Figure 6.
AMPAR antagonism impairs behavioral recovery. Loss of behavioral recovery was assessed after administration of an AMPA receptor selective agonist, CFM2 (50 μmol/kg), on grid-walking (A, B), cylinder/forelimb asymmetry (C), and reaching (D) tasks. Treatment with CFM2 resulted in a significant increase in the number of footfaults on the grid-walking task (A) and a decrease in the number of pellets successfully retrieved on the reaching task (D). Data are shown as mean ± SEM for n = 10 per group. *p < 0.05, **p < 0.01, ***p < 0.001 compared with sham controls; #p < 0.05 compared with stroke plus vehicle-treated animals.
Figure 7.
BDA injection volume and location are uniform across experimental groups. There were no significant differences between the number of BDA-labeled cell bodies, BDA volumes, and location between stroke plus vehicle and stroke plus CX1837-treated animals (A). Photomicrographs show representative BDA injection sizes for three animals for stroke plus vehicle and stroke plus CX1837 (B). Sample photomicrographs show representative imaged of BDA-labeled cell bodies in somatosensory cortex (C). Data shown are averages ± SEM for n = 4 per group.
Figure 8.
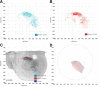
Patterns of cortical connections in control and in conditions of AMPAR conductance. A small injection of the neuroanatomical tracer BDA was placed into the forelimb motor cortex adjacent to the stroke site 6 weeks after stroke. The location of all labeled cell bodies in the forelimb motor cortex, forelimb and hindlimb somatosensory cortex, and facial (whisker) somatosensory cortex were digitally plotted. These plots convert the location of all the axonal connections of forelimb motor cortex into x/y plots, which are then grouped according to treatment condition and statistically compared among groups (Hotelling's inverse T matrix). The plots in A (stroke plus vehicle treatment) and B (stroke plus CX1837 treatment) show the location of labeled axons in groups of animals (n = 4 for each condition). For CX1837-treated mice, there is no difference in the spatial distribution (C) relative to vehicle-treated stroke controls. Polar distribution plots, incorporating normalized axon quantity and distribution of axons in register with connectional plot (D). Shaded polygons (D) represent 70th percentile of the distances of labeled axons from the injection site in each segment of the graph.
Figure 9.
Inflection point in CX1837 effect on infarct size. Representative Nissl-stained sections 7 d after stroke from stroke plus vehicle treatment (A), stroke plus CX1837 treatment starting at the time of stroke (B), and stroke plus CX1837 treatment starting from 5 d after insult (C). Quantification of the stroke volume is shown in D. Data are shown as mean ± SEM for n = 4 per group. *p < 0.05.
Similar articles
- Ampakines cause sustained increases in brain-derived neurotrophic factor signaling at excitatory synapses without changes in AMPA receptor subunit expression.
Lauterborn JC, Pineda E, Chen LY, Ramirez EA, Lynch G, Gall CM. Lauterborn JC, et al. Neuroscience. 2009 Mar 3;159(1):283-95. doi: 10.1016/j.neuroscience.2008.12.018. Epub 2008 Dec 24. Neuroscience. 2009. PMID: 19141314 Free PMC article. - Brain-derived neurotrophic factor rapidly increases AMPA receptor surface expression in rat nucleus accumbens.
Li X, Wolf ME. Li X, et al. Eur J Neurosci. 2011 Jul;34(2):190-8. doi: 10.1111/j.1460-9568.2011.07754.x. Epub 2011 Jun 21. Eur J Neurosci. 2011. PMID: 21692887 Free PMC article. - BDNF-induced synaptic delivery of AMPAR subunits is differentially dependent on NMDA receptors and requires ERK.
Li W, Keifer J. Li W, et al. Neurobiol Learn Mem. 2009 Mar;91(3):243-9. doi: 10.1016/j.nlm.2008.10.002. Epub 2008 Nov 17. Neurobiol Learn Mem. 2009. PMID: 18977306 Free PMC article. - Promoting neuroplasticity for motor rehabilitation after stroke: considering the effects of aerobic exercise and genetic variation on brain-derived neurotrophic factor.
Mang CS, Campbell KL, Ross CJ, Boyd LA. Mang CS, et al. Phys Ther. 2013 Dec;93(12):1707-16. doi: 10.2522/ptj.20130053. Epub 2013 Aug 1. Phys Ther. 2013. PMID: 23907078 Free PMC article. Review. - Regulation of neuronal PKA signaling through AKAP targeting dynamics.
Dell'Acqua ML, Smith KE, Gorski JA, Horne EA, Gibson ES, Gomez LL. Dell'Acqua ML, et al. Eur J Cell Biol. 2006 Jul;85(7):627-33. doi: 10.1016/j.ejcb.2006.01.010. Epub 2006 Feb 28. Eur J Cell Biol. 2006. PMID: 16504338 Review.
Cited by
- Traumatic brain injury: molecular biomarkers, genetics, secondary consequences, and medical management.
Lipsky RH, Witkin JM, Shafique H, Smith JL, Cerne R, Marini AM. Lipsky RH, et al. Front Neurosci. 2024 Oct 4;18:1446076. doi: 10.3389/fnins.2024.1446076. eCollection 2024. Front Neurosci. 2024. PMID: 39450122 Free PMC article. Review. - Delayed administration of a small molecule tropomyosin-related kinase B ligand promotes recovery after hypoxic-ischemic stroke.
Han J, Pollak J, Yang T, Siddiqui MR, Doyle KP, Taravosh-Lahn K, Cekanaviciute E, Han A, Goodman JZ, Jones B, Jing D, Massa SM, Longo FM, Buckwalter MS. Han J, et al. Stroke. 2012 Jul;43(7):1918-24. doi: 10.1161/STROKEAHA.111.641878. Epub 2012 Apr 24. Stroke. 2012. PMID: 22535263 Free PMC article. - Translating concepts of neural repair after stroke: Structural and functional targets for recovery.
Regenhardt RW, Takase H, Lo EH, Lin DJ. Regenhardt RW, et al. Restor Neurol Neurosci. 2020;38(1):67-92. doi: 10.3233/RNN-190978. Restor Neurol Neurosci. 2020. PMID: 31929129 Free PMC article. Review. - Pharmacological Enhancement of Stroke Recovery.
Kumar A, Kitago T. Kumar A, et al. Curr Neurol Neurosci Rep. 2019 May 30;19(7):43. doi: 10.1007/s11910-019-0959-2. Curr Neurol Neurosci Rep. 2019. PMID: 31144053 Review. - Melatonin receptor agonist ramelteon attenuates mouse acute and chronic ischemic brain injury.
Wu XL, Lu SS, Liu MR, Tang WD, Chen JZ, Zheng YR, Ahsan A, Cao M, Jiang L, Hu WW, Wu JY, Chen Z, Zhang XN. Wu XL, et al. Acta Pharmacol Sin. 2020 Aug;41(8):1016-1024. doi: 10.1038/s41401-020-0361-2. Epub 2020 Feb 27. Acta Pharmacol Sin. 2020. PMID: 32107468 Free PMC article.
References
- Arai AC, Kessler M. Pharmacology of ampakine modulators: from AMPA receptors to synapses and behavior. Curr Drug Targets. 2007;8:583–602. - PubMed
- Batchelor PE, Wills TE, Hewa AP, Porritt MJ, Howells DW. Stimulation of axonal sprouting by trophic factors immobilized within the wound core. Brain Res. 2008;1209:49–56. - PubMed
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. - PubMed
- Bramham CR. Local protein synthesis, actin dynamics, and LTP consolidation. Curr Opin Neurobiol. 2008;18:524–531. - PubMed
- Braun JS, Jander S, Schroeter M, Witte OW, Stoll G. Spatiotemporal relationship of apoptotic cell death to lymphomonocytic infiltration in photochemically induced focal ischemia of the rat cerebral cortex. Acta Neuropathol. 1996;92:255–263. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical