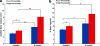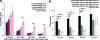Effect of polycaprolactone scaffold permeability on bone regeneration in vivo - PubMed (original) (raw)
Effect of polycaprolactone scaffold permeability on bone regeneration in vivo
Anna G Mitsak et al. Tissue Eng Part A. 2011 Jul.
Abstract
Successful bone tissue engineering depends on the scaffold's ability to allow nutrient diffusion to and waste removal from the regeneration site, as well as provide an appropriate mechanical environment. Since bone is highly vascularized, scaffolds that provide greater mass transport may support increased bone regeneration. Permeability encompasses the salient features of three-dimensional porous scaffold architecture effects on scaffold mass transport. We hypothesized that higher permeability scaffolds will enhance bone regeneration for a given cell seeding density. We manufactured poly-ɛ-caprolactone scaffolds, designed to have the same internal pore design and either a low permeability (0.688×10(-7)m(4)/N-s) or a high permeability (3.991×10(-7)m(4)/N-s), respectively. Scaffolds were seeded with bone morphogenic protein-7-transduced human gingival fibroblasts and implanted subcutaneously in immune-compromised mice for 4 and 8 weeks. Micro-CT evaluation showed better bone penetration into high permeability scaffolds, with blood vessel infiltration visible at 4 weeks. Compression testing showed that scaffold design had more influence on elastic modulus than time point did and that bone tissue infiltration increased the mechanical properties of the high permeability scaffolds at 8 weeks. These results suggest that for polycaprolactone, a more permeable scaffold with regular architecture is best for in vivo bone regeneration. This finding is an important step toward the end goal of optimizing a scaffold for bone tissue engineering.
Figures
FIG. 1.
(a) Bone volume (BV) inside the scaffold ROI at 4 and 8 weeks. (b) Bone in-growth for the entire scaffold ROI at 4 and 8 weeks. *_p_≤0.05. **_p_≤0.01. ROI, region of interest. Color images available online at
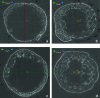
FIG. 2.
Micro-CT image slices from the center of representative low and high permeability scaffolds. (a) Low permeability scaffold at 4 weeks, (b) low permeability scaffold at 8 weeks, (c) high permeability scaffold at 4 weeks, and (d) high permeability scaffold at 8 weeks. Color images available online at
FIG. 3.
(a) Tissue mineral content (TMC) and (b) tissue mineral density (TMD) at 4 and 8 weeks. *_p_≤0.05. Color images available online at
FIG. 4.
Micro-CT slice images showing top-down views of the cylindrical ROIs used for the concentric BV analysis. (a–d) show the four concentric ROIs, each with a progressively smaller outer diameter, and (e) shows all four ROIs overlaid on one another. Color images available online at
FIG. 5.
Concentric cylinder BV analysis. (a) BV and (b) bone in-growth for high and low permeability scaffolds and 4 and 8 weeks, for four hollow, cylindrical ROIs. **_p_≤0.01. Color images available online at
FIG. 6.
(a) Tangent modulus at 12.5% strain, 4 and 8 weeks. (b) Compressive yield strength at 0.2% offset. *_p_≤0.05. **_p_≤0.01. Color images available online at
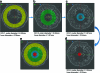
FIG. 7.
Representative histological sections of high permeability (a, b) and low permeability (c, d) scaffolds at 8 weeks. (b) and (d) are higher magnification images of rectangular insets indicated in (a) and (c). The scaffold is indicated by “S” and bone growth in and around the scaffold is indicated by the dark pink staining and arrows. Osteocytes in lacunae are seen in both high and low permeability scaffolds (indicated by dashed circles) as is marrow space (“M”). Blood vessels are also seen in the high permeability scaffold (BV). Color images available online at
Similar articles
- The pore size of polycaprolactone scaffolds has limited influence on bone regeneration in an in vivo model.
Roosa SM, Kemppainen JM, Moffitt EN, Krebsbach PH, Hollister SJ. Roosa SM, et al. J Biomed Mater Res A. 2010 Jan;92(1):359-68. doi: 10.1002/jbm.a.32381. J Biomed Mater Res A. 2010. PMID: 19189391 - Effects of designed PLLA and 50:50 PLGA scaffold architectures on bone formation in vivo.
Saito E, Liao EE, Hu WW, Krebsbach PH, Hollister SJ. Saito E, et al. J Tissue Eng Regen Med. 2013 Feb;7(2):99-111. doi: 10.1002/term.497. Epub 2011 Dec 9. J Tissue Eng Regen Med. 2013. PMID: 22162220 Free PMC article. - Biomineral coating increases bone formation by ex vivo BMP-7 gene therapy in rapid prototyped poly(L-lactic acid) (PLLA) and poly(ε-caprolactone) (PCL) porous scaffolds.
Saito E, Suarez-Gonzalez D, Murphy WL, Hollister SJ. Saito E, et al. Adv Healthc Mater. 2015 Mar 11;4(4):621-32. doi: 10.1002/adhm.201400424. Epub 2014 Dec 16. Adv Healthc Mater. 2015. PMID: 25515846 - Role of pore size and morphology in musculo-skeletal tissue regeneration.
Perez RA, Mestres G. Perez RA, et al. Mater Sci Eng C Mater Biol Appl. 2016 Apr 1;61:922-39. doi: 10.1016/j.msec.2015.12.087. Epub 2015 Dec 31. Mater Sci Eng C Mater Biol Appl. 2016. PMID: 26838923 Review. - Advancements in gradient bone scaffolds: enhancing bone regeneration in the treatment of various bone disorders.
Zhen C, Shi Y, Wang W, Zhou G, Li H, Lin G, Wang F, Tang B, Li X. Zhen C, et al. Biofabrication. 2024 May 13;16(3). doi: 10.1088/1758-5090/ad4595. Biofabrication. 2024. PMID: 38688259 Review.
Cited by
- Porous Scaffold Design for Additive Manufacturing in Orthopedics: A Review.
Chen H, Han Q, Wang C, Liu Y, Chen B, Wang J. Chen H, et al. Front Bioeng Biotechnol. 2020 Jun 17;8:609. doi: 10.3389/fbioe.2020.00609. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32626698 Free PMC article. Review. - Pore architecture effects on chondrogenic potential of patient-specific 3-dimensionally printed porous tissue bioscaffolds for auricular tissue engineering.
Zopf DA, Flanagan CL, Mitsak AG, Brennan JR, Hollister SJ. Zopf DA, et al. Int J Pediatr Otorhinolaryngol. 2018 Nov;114:170-174. doi: 10.1016/j.ijporl.2018.07.033. Epub 2018 Jul 24. Int J Pediatr Otorhinolaryngol. 2018. PMID: 30262359 Free PMC article. - An overview of 3D printed metal implants in orthopedic applications: Present and future perspectives.
Wu Y, Liu J, Kang L, Tian J, Zhang X, Hu J, Huang Y, Liu F, Wang H, Wu Z. Wu Y, et al. Heliyon. 2023 Jun 29;9(7):e17718. doi: 10.1016/j.heliyon.2023.e17718. eCollection 2023 Jul. Heliyon. 2023. PMID: 37456029 Free PMC article. Review. - Citrate-modified bacterial cellulose as a potential scaffolding material for bone tissue regeneration.
Salihu R, Abd Razak SI, Sani MH, Wsoo MA, Zawawi NA, Shahir S. Salihu R, et al. PLoS One. 2024 Dec 31;19(12):e0312396. doi: 10.1371/journal.pone.0312396. eCollection 2024. PLoS One. 2024. PMID: 39739716 Free PMC article. - A Review on Properties of Natural and Synthetic Based Electrospun Fibrous Materials for Bone Tissue Engineering.
Bhattarai DP, Aguilar LE, Park CH, Kim CS. Bhattarai DP, et al. Membranes (Basel). 2018 Aug 14;8(3):62. doi: 10.3390/membranes8030062. Membranes (Basel). 2018. PMID: 30110968 Free PMC article. Review.
References
- Karande T.S. Ong J.L. Agrawal C.M. Diffusion in musculoskeletal tissue engineering scaffolds: design issues related to porosity, permeability, and nutrient mixing. Ann Biomed Eng. 2004;32:1728. - PubMed
- Jones A.C. Arns C.H. Hutmacher D.W. Milthorpe B.K. Sheppard A.P. Knackstedt M.A. The correlation of pore morphology, interconnectivity and physical properties of 3D ceramic scaffolds with bone ingrowth. Biomaterials. 2009;30:1440. - PubMed
- Lee K.E. Wang S. Fox B.C. Ritman E.L. Yaszemski M.J. Lu L. Poly(propylene fumurate) bone tissue engineering scaffold fabrication using stereolithography: effects of resin formulations and laser parameters. Biomacromolecules. 2007;8:1077. - PubMed
- Wang K. Cai L. Hao F. Xu X. Cui M. Wang S. Distinct cell responses to substrates consisting of poly-ɛ-caprolactone and poly(propylene fumurate) in the presence or absence of cross-links. Biomacromolecules. 2010;11:2748. - PubMed
- Ciapetti G. Ambrosio L. Savarino L. Granchi D. Cenni E. Baldini N., et al. Osteoblast growth and function in porous poly-ɛ-caprolactone matrices for bone repair: a preliminary study. Biomaterials. 2003;24:3815. - PubMed