Glutathionylation of peroxiredoxin I induces decamer to dimers dissociation with concomitant loss of chaperone activity - PubMed (original) (raw)
. 2011 Apr 19;50(15):3204-10.
doi: 10.1021/bi101373h. Epub 2011 Mar 25.
Affiliations
- PMID: 21401077
- PMCID: PMC3176717
- DOI: 10.1021/bi101373h
Glutathionylation of peroxiredoxin I induces decamer to dimers dissociation with concomitant loss of chaperone activity
Ji Won Park et al. Biochemistry. 2011.
Abstract
Reversible protein glutathionylation, a redox-sensitive regulatory mechanism, plays a key role in cellular regulation and cell signaling. Peroxiredoxins (Prxs), a family of peroxidases that is involved in removing H(2)O(2) and organic hydroperoxides, are known to undergo a functional change from peroxidase to molecular chaperone upon overoxidation of its catalytic cysteine. The functional change is caused by a structural change from low molecular weight oligomers to high molecular weight complexes that possess molecular chaperone activity. We reported earlier that Prx I can be glutathionylated at three of its cysteine residues, Cys52, -83, and -173 [Park et al. (2009) J. Biol. Chem., 284, 23364]. In this study, using analytical ultracentrifugation analysis, we reveal that glutathionylation of Prx I, WT, or its C52S/C173S double mutant shifted its oligomeric status from decamers to a population consisting mainly of dimers. Cys83 is localized at the putative dimer-dimer interface, implying that the redox status of Cys83 may play an important role in stabilizing the oligomeric state of Prx I. Studies with the Prx I (C83S) mutant show that while Cys83 is not essential for the formation of high molecular weight complexes, it affects the dimer-decamer equilibrium. Glutathionylation of the C83S mutant leads to accumulation of dimers and monomers. In addition, glutathionylation of Prx I, both the WT and C52S/C173S mutants, greatly reduces their molecular chaperone activity in protecting citrate synthase from thermally induced aggregation. Together, these results reveal that glutathionylation of Prx I promotes changes in its quaternary structure from decamers to smaller oligomers and concomitantly inactivates its molecular chaperone function.
Figures
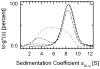
Fig. 1. Concentration dependence of sedimentation velocity profiles for purified WT Prx I
The normalized ls-g*(s), obtained as described in Experimental Procedures, was plotted as a function of sedimentation coefficients corrected to s20,w values. The concentrations of WT Prx I used were: 50 μM (solid line), 5 μM (dashed line), 2 μM (dash-dot-dot-dashed line), and 0.2 μM (short dashed line)
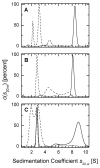
Fig. 2. Analysis of the oligomeric status of Prx I by sedimentation velocity methods
Sedimentation coefficients distributions were corrected to standard conditions and the c(s20,w) profiles were plotted as a function of sedimentation coefficient. Experiments with glutathionylated proteins at 50 μM were carried out in a buffer containing 10 mM GSSG (see Experimental Procedures). (A) sedimentation coefficient distribution of reduced (solid line) and glutathionylated (dashed line) WT Prx I, (B) reduced (solid line) and glutathionylated (dashed line) Prx I (C52S/C173S) double mutant, (C) 50 μM reduced (solid line), 10 μM reduced (dash-dot-dashed line), and glutathionylated (dashed line) Prx I (C83S) mutant.
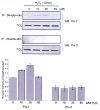
Fig. 3. Glutathionylation of Prx I and II in H2O2–treated HeLa cells
To induce glutathionylation with biotinylated glutathione, cells were preincubated with 250 μM BioGEE for 1 h and subsequently exposed to indicated concentration of H2O2 for 10 min. Proteins covalently bound to biotin were extracted using streptavidin agarose then eluted with DTT and subjected to western blot analysis with anti-Prx I (upper panel) or anti-Prx II (middle panel) antibody. Histogram (lower panel) depicts as means +/− S.E. (n=3) from the densitometric analysis.
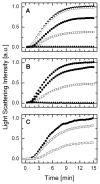
Fig. 4. Glutathionylation induces a substantial reduction in chaperone activity of Prx I and its (C52/173S) mutant
Chaperone activity of Prx I was monitored by its ability to protect citrate synthase (CS) from thermally induced aggregation. In these experiments, aggregation of 2 μM of CS at 45°C, pH 7.4, was monitored either alone or in the presence of 10 μM deglutathionylated or glutathionylated Prx I. (A) Effect of WT Prx I. Solid circle, 2 μM CS alone; open circle, 2 μM CS plus 10 mM GSSG; open square, 2 μM CS plus 10 μM WT Prx I; solid square, 2 μM CS plus 10 μM glutathionylated WT Prx I; solid triangle, 10 μM WT Prx I alone. (B) Effect of Prx I (C52S/C173S): Solid circle, 2 μM CS alone; open square, 2 μM CS plus 10 μM Prx I (C52S/C173S); solid square, 2 μM CS plus 10 μM glutathionylated Prx I (C52S/C173S); solid triangle, 10 μM Prx I (C52S/C173S) alone. (C) Effect of Prx I (C83S): Solid circle, 2 μM CS alone; open diamond, 2 μM CS plus 10 μM Prx I (C83S); open square, 2 μM CS plus 10 μM WT Prx I.
Similar articles
- Protein glutathionylation in the regulation of peroxiredoxins: a family of thiol-specific peroxidases that function as antioxidants, molecular chaperones, and signal modulators.
Chae HZ, Oubrahim H, Park JW, Rhee SG, Chock PB. Chae HZ, et al. Antioxid Redox Signal. 2012 Mar 15;16(6):506-23. doi: 10.1089/ars.2011.4260. Antioxid Redox Signal. 2012. PMID: 22114845 Free PMC article. Review. - Site-directed mutagenesis substituting cysteine for serine in 2-Cys peroxiredoxin (2-Cys Prx A) of Arabidopsis thaliana effectively improves its peroxidase and chaperone functions.
Lee EM, Lee SS, Tripathi BN, Jung HS, Cao GP, Lee Y, Singh S, Hong SH, Lee KW, Lee SY, Cho JY, Chung BY. Lee EM, et al. Ann Bot. 2015 Sep;116(4):713-25. doi: 10.1093/aob/mcv094. Epub 2015 Jul 2. Ann Bot. 2015. PMID: 26141131 Free PMC article. - Switching between the alternative structures and functions of a 2-Cys peroxiredoxin, by site-directed mutagenesis.
Angelucci F, Saccoccia F, Ardini M, Boumis G, Brunori M, Di Leandro L, Ippoliti R, Miele AE, Natoli G, Scotti S, Bellelli A. Angelucci F, et al. J Mol Biol. 2013 Nov 15;425(22):4556-68. doi: 10.1016/j.jmb.2013.09.002. Epub 2013 Sep 8. J Mol Biol. 2013. PMID: 24021815 - Dimer-oligomer interconversion of wild-type and mutant rat 2-Cys peroxiredoxin: disulfide formation at dimer-dimer interfaces is not essential for decamerization.
Matsumura T, Okamoto K, Iwahara SI, Hori H, Takahashi Y, Nishino T, Abe Y. Matsumura T, et al. J Biol Chem. 2008 Jan 4;283(1):284-293. doi: 10.1074/jbc.M705753200. Epub 2007 Nov 1. J Biol Chem. 2008. PMID: 17974571 - Hyperoxidation of Peroxiredoxins: Gain or Loss of Function?
Veal EA, Underwood ZE, Tomalin LE, Morgan BA, Pillay CS. Veal EA, et al. Antioxid Redox Signal. 2018 Mar 1;28(7):574-590. doi: 10.1089/ars.2017.7214. Epub 2017 Sep 8. Antioxid Redox Signal. 2018. PMID: 28762774 Review.
Cited by
- Glutathione synthesis and its role in redox signaling.
Zhang H, Forman HJ. Zhang H, et al. Semin Cell Dev Biol. 2012 Sep;23(7):722-8. doi: 10.1016/j.semcdb.2012.03.017. Epub 2012 Apr 3. Semin Cell Dev Biol. 2012. PMID: 22504020 Free PMC article. Review. - Real-time monitoring of peroxiredoxin oligomerization dynamics in living cells.
Pastor-Flores D, Talwar D, Pedre B, Dick TP. Pastor-Flores D, et al. Proc Natl Acad Sci U S A. 2020 Jul 14;117(28):16313-16323. doi: 10.1073/pnas.1915275117. Epub 2020 Jun 29. Proc Natl Acad Sci U S A. 2020. PMID: 32601209 Free PMC article. - Differential Kinetics of Two-Cysteine Peroxiredoxin Disulfide Formation Reveal a Novel Model for Peroxide Sensing.
Portillo-Ledesma S, Randall LM, Parsonage D, Dalla Rizza J, Karplus PA, Poole LB, Denicola A, Ferrer-Sueta G. Portillo-Ledesma S, et al. Biochemistry. 2018 Jun 19;57(24):3416-3424. doi: 10.1021/acs.biochem.8b00188. Epub 2018 Mar 30. Biochemistry. 2018. PMID: 29553725 Free PMC article. - The ascorbate-glutathione cycle coming of age.
Foyer CH, Kunert K. Foyer CH, et al. J Exp Bot. 2024 May 3;75(9):2682-2699. doi: 10.1093/jxb/erae023. J Exp Bot. 2024. PMID: 38243395 Free PMC article. Review. - A primer on peroxiredoxin biochemistry.
Karplus PA. Karplus PA. Free Radic Biol Med. 2015 Mar;80:183-90. doi: 10.1016/j.freeradbiomed.2014.10.009. Epub 2014 Oct 19. Free Radic Biol Med. 2015. PMID: 25452140 Free PMC article. Review.
References
- Hwang C, Sinskey AJ, Lodish HF. Oxidized redox state of glutathione in the endoplasmic reticulum. Science. 1992;257:1496–1502. - PubMed
- Gilbert HF. Thiol/disulfide exchange equilibria and disulfide bond stability. Methods Enzymol. 1995;251:8–28. - PubMed
- Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 1995;270:296–299. - PubMed
- Bae YS, Kang SW, Seo MS, Baines IC, Tekle E, Chock PB, Rhee SG. Epidermal growth factor (EGF)-induced generation of hydrogen peroxide. Role in EGF receptor-mediated tyrosine phosphorylation. J Biol Chem. 1997;272:217–221. - PubMed
- Barrett WC, DeGnore JP, Keng YF, Zhang ZY, Yim MB, Chock PB. Roles of superoxide radical anion in signal transduction mediated by reversible regulation of protein-tyrosine phosphatase 1B. J Biol Chem. 1999;274:34543–34546. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources