Phosphatidylserine is a critical modulator for Akt activation - PubMed (original) (raw)
Phosphatidylserine is a critical modulator for Akt activation
Bill X Huang et al. J Cell Biol. 2011.
Abstract
Akt activation relies on the binding of Akt to phosphatidylinositol-3,4,5-trisphosphate (PIP(3)) in the membrane. Here, we demonstrate that Akt activation requires not only PIP(3) but also membrane phosphatidylserine (PS). The extent of insulin-like growth factor-induced Akt activation and downstream signaling as well as cell survival under serum starvation conditions positively correlates with plasma membrane PS levels in living cells. PS promotes Akt-PIP(3) binding, participates in PIP(3)-induced Akt interdomain conformational changes for T308 phosphorylation, and causes an open conformation that allows for S473 phosphorylation by mTORC2. PS interacts with specific residues in the pleckstrin homology (PH) and regulatory (RD) domains of Akt. Disruption of PS-Akt interaction by mutation impairs Akt signaling and increases susceptibility to cell death. These data identify a critical function of PS for Akt activation and cell survival, particularly in conditions with limited PIP(3) availability. The novel molecular interaction mechanism for Akt activation suggests potential new targets for controlling Akt-dependent cell survival and proliferation.
Figures
Figure 1.
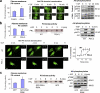
Effects of plasma membrane PS on membrane translocation of Akt-PH domain and Akt activation. (a) Increasing plasma membrane PS in Neuro 2A cells by DHA supplementation facilitated IGF-induced Akt membrane translocation and phosphorylation. The membrane translocation is depicted by a representative fluorescence intensity profile across a transverse section in indicated cells (FI). (b) Decreasing plasma membrane PS using PSA-3 mutant CHO cells impaired Akt-PH domain translocation and Akt phosphorylation without affecting PI3 kinase activity. Shown are representative micrographs, time course of translocation by using averaged relative R values, and PI3 kinase activity. (c) Increasing plasma membrane PS by expressing the PSS1(R95K) facilitated IGF-induced Akt activation without altering PI3 kinase activity in Neuro 2A cells. GFP-PSS1 was observed at the apparent molecular mass of ~68 kD. The dimeric form of GFP (∼52 kD) was detected in samples expressing GFP alone. The plasma membrane PS contents are expressed as mean ± SD (error bars; n = 3), representing two independent experiments. *, P < 0.05. Bars, 10 µm.
Figure 2.
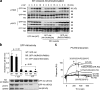
Effects of membrane phospholipids on interdomain conformational changes of Akt due to membrane interaction probed by mass spectrometry. The appearance of two interdomain cross-linked peptides (K30-K389 and K284-K426) depended on the phospholipid composition of interacting membranes. Cross-linked peptides are marked with asterisks (left). The zoomed-in-views (middle) show quantitative comparison of these cross-linked peptides using 16O/18O labeling as described in Materials and methods. Although the16O/18O ratio from non–cross-linked peptide pairs such as T[87–111]K (2886 D vs. 2990 D) remained the same, the 16O/18O ratio from the interdomain cross-linked peptide pairs separated by 8 D changed according to the membrane phospholipid composition. Schematic presentations of the interdomain conformational changes of Akt deduced by the cross-linking data are shown (right).
Figure 3.
Effects of phospholipids on the membrane interaction and phosphorylation of Akt. (a) Representative SPR sensorgrams of the binding of full-length Akt to liposomes with various phospholipid compositions (x:y:50:[50 − x − y] PIP3/PS/PE/PC). 200 nM Akt was injected onto the L1 chip coated with liposomes. The response of the control liposomes containing PE/PC (50:50), which was insignificant, was subtracted from each sensorgram to correct for background drift. The sensorgram was obtained using PBS as the running buffer. (b) The effect of salt concentration on the binding of Akt to liposomes containing PIP3 and/or PS. (c–e) Representative SPR sensorgrams showing insignificant effects of PIP2, PIP (c), PI (d), and PE/PC on Akt-membrane interaction. (f) Western blot data showing PS- or PIP3-dependent Akt phosphorylation of T308 and S473 by PDK1 and mTORC2, respectively. The band intensity of pT308 or pS473 were normalized to the Akt level (data are mean ± SD [error bars], n ≥ 3). Statistical significance was tested against liposomes containing 0% PS and 0% PIP3. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Figure 4.
Membrane interaction of the individual domains of Akt affected by PIP3 and PS. SPR sensorgrams representing the membrane binding of Akt RD (a), PH domain (b), and PH domain mutants (c). The GFP-RD or GFP-PH domain of Akt was captured by anti-GFP antibody immobilized on a CM5 chip followed by the injection of liposomes containing PIP3/PS/PE/PC (x:y:50:[50 − x − y]). Both RD and PH domains showed PS-dependent binding. PH domain mutation (R15A or K20A) significantly impairs the Akt binding to membrane without affecting Akt-PIP3 interaction (c). Inset, SPR sensorgram showing negligible binding of liposomes to GFP alone.
Figure 5.
Akt membrane translocation and phosphorylation impaired by disrupting Akt–PS interaction via mutation of the PS-binding residues in the PH domain. (a) Representative micrographs showing IGF-induced membrane translocation of Neuro 2A cells coexpressing RFP-PH (WT) and GFP-PH (R15A or K20A). The membrane translocation is depicted by a representative fluorescence intensity profile across a transverse section in indicated cells (FI). Bars, 10 µm. (b) Time course of IGF-induced Akt phosphorylation at T308 and S473 in Neuro 2A cells expressing GFP-Akt WT or GFP-Akt mutants. (c) Computer modeling depicting binding of PS to R15 and K20 located outside the PIP3-binding pocket in the PH domain. The broken line indicates a putative membrane surface. The lowest energy conformations generated by using a Lamarckian genetic algorithm (Morris et al., 1998) showed the putative binding of PS to R15 and K20 via hydrogen bonding.
Figure 6.
Akt activation impaired by disrupting Akt–PS interaction via mutation of the PS-binding residues in the RD. (a) Time course of IGF-induced Akt phosphorylation at T308 and S473 in Neuro 2A cells expressing GFP-Akt WT or full-length GFP-Akt mutants. Mutation of the K419A/K420A impaired Akt phosphorylation, particularly at S473 located in the RD region. (b) Kinase activity of GFP-Akt WT or mutants immunoprecipitated from Neuro 2A cell lysates. Representative Western blotting data are shown to indicate the IGF-induced phosphorylation status of GFP-Akt or mutants in the lysate and the amount of GFP-Akt or mutants immunoprecipitated on the beads. Error bars indicate ± SD. (c) The effect of the mutation on the binding of Akt-RD to membrane PS. Statistical significance was tested against GFP-Akt WT without IGF-stimulation unless indicated. * or **, significant increase (*, P < 0.05; **, P < 0.01); #, significant decrease (P < 0.05).
Figure 7.
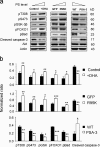
Effects of mutation of PS-binding residues on Akt downstream signaling and apoptotic cell death induced by serum starvation. Neuro 2A cells were transfected with GFP-Akt WT or mutants for 36 h and subjected to serum starvation for 48 h. (a) GFP-Akt (R15A) or GFP-Akt (K20A) expressed in Neuro 2A cells showed significantly impaired phosphorylation. Phosphorylation of GSK-3β, FOXO1, and Bad, the downstream signaling of the Akt activation, was also reduced in cells expressing these mutants, with a concomitant increase in active caspase-3. (b) Cells expressing the mutants showed significantly more TUNEL-positive cells (green + red = orange–∼yellow) in comparison to cells expressing GFP-Akt WT. TUNEL-positive cells were stained red using Click-iT TUNEL assay kit with Alexa Fluor 594 Azide. Nuclei were stained with Hoechst 33342. Bar, 30 µm (c) The percentages of GFP- and TUNEL-positive cells were determined by counting the total of 500–1,000 GFP-Akt WT or GFP-Akt mutant expressing cells from six randomly selected fields. Inset, Western blot analysis indicating comparable expression of WT and mutant GFP-Akt. Results represent two independent experiments performed in duplicates. Serum-sufficient cells transfected with WT or mutants showed negligible cell death. Statistical analysis was performed by post-hoc Tukey honestly significant difference test at the significance level of P < 0.05. Different letters indicate statistically significant differences.
Figure 8.
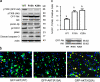
Effects of plasma membrane PS on Akt activation, downstream signaling, and apoptotic cell death induced by serum starvation. (a) Increasing plasma membrane PS in Neuro 2A cells by DHA supplementation or expressing the PSS1 (R95K) mutant enhanced phosphorylation of Akt and its downstream targets, resulting in less caspase-3 activation. In contrast, the PSA-3 mutant, where the PS level is lower in comparison to the WT, showed increased caspase-3 activation with decreased Akt signaling. (b) Quantitative analysis of the Western blot data. The band intensity was normalized to either Akt (pT308 and pS473) or actin level (pGSK-3β, pFOXO1, pBad, and active caspase-3). *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Figure 9.
Schematic presentation of the PS involvement in Akt activation. PS and PIP3 jointly regulate the membrane binding and interdomain conformational changes of Akt for unfolding the PH and RD to expose T308 and S473 for phosphorylation and activation. When the growth factor receptor is stimulated, PIP3 is generated in the membrane, which in turn triggers translocation of cytosolic Akt to the plasma membrane through the specific binding of PIP3 to the PH domain. Although necessary, the PIP3–PH interaction alone is not sufficient for securing Akt binding to the plasma membrane. Interaction of PS with the PS-binding residues in the PH domain outside the PIP3-binding pocket is also required for the membrane binding and conformational changes to expose T308 for phosphorylation by PDK1. Near the plasma membrane, presumably after or concurrent with PIP3–PH binding, the PS-binding residues in the RD also interact with PS, resulting in an open conformation, allowing S473 phosphorylation by mTORC2. Although direct binding of PIP3 with the RD is minimal, PIP3 can also induce an open RD conformation to expose S473 for phosphorylation. The involvement of KD has not been evaluated separately.
Similar articles
- Threonine 34 phosphorylation by phosphoinositide-dependent protein kinase 1 facilitates dissociation of Akt from the plasma membrane.
Huang BX, Lee R, Akbar M, Kim HY. Huang BX, et al. Int J Biochem Cell Biol. 2015 Jul;64:195-201. doi: 10.1016/j.biocel.2015.04.007. Epub 2015 Apr 22. Int J Biochem Cell Biol. 2015. PMID: 25912234 Free PMC article. - Postreceptoral adipocyte insulin resistance induced by nelfinavir is caused by insensitivity of PKB/Akt to phosphatidylinositol-3,4,5-trisphosphate.
Kachko I, Maissel A, Mazor L, Ben-Romano R, Watson RT, Hou JC, Pessin JE, Bashan N, Rudich A. Kachko I, et al. Endocrinology. 2009 Jun;150(6):2618-26. doi: 10.1210/en.2008-1205. Epub 2009 Jan 29. Endocrinology. 2009. PMID: 19179444 Free PMC article. - Effects of ethanol on conformational changes of Akt studied by chemical cross-linking, mass spectrometry, and (18)O labeling.
Huang BX, Kim HY. Huang BX, et al. ACS Chem Biol. 2012 Feb 17;7(2):387-94. doi: 10.1021/cb2003237. Epub 2011 Dec 7. ACS Chem Biol. 2012. PMID: 22129086 Free PMC article. - Disruption of the interface between the pleckstrin homology (PH) and kinase domains of Akt protein is sufficient for hydrophobic motif site phosphorylation in the absence of mTORC2.
Warfel NA, Niederst M, Newton AC. Warfel NA, et al. J Biol Chem. 2011 Nov 11;286(45):39122-9. doi: 10.1074/jbc.M111.278747. Epub 2011 Sep 9. J Biol Chem. 2011. PMID: 21908613 Free PMC article. - Targeting WNT, protein kinase B, and mitochondrial membrane integrity to foster cellular survival in the nervous system.
Chong ZZ, Maiese K. Chong ZZ, et al. Histol Histopathol. 2004 Apr;19(2):495-504. doi: 10.14670/HH-19.495. Histol Histopathol. 2004. PMID: 15024710 Free PMC article. Review.
Cited by
- Can calmodulin bind to lipids of the cytosolic leaflet of plasma membranes?
Scollo F, Tempra C, Evci H, Riopedre-Fernandez M, Olżyńska A, Javanainen M, Uday A, Cebecauer M, Cwiklik L, Martinez-Seara H, Jungwirth P, Jurkiewicz P, Hof M. Scollo F, et al. Open Biol. 2024 Sep;14(9):240067. doi: 10.1098/rsob.240067. Epub 2024 Sep 18. Open Biol. 2024. PMID: 39288811 Free PMC article. - Attenuation of PI3K/AKT signaling pathway by Ocimum gratissimum leaf flavonoid-rich extracts in streptozotocin-induced diabetic male rats.
Ajiboye BO, Famusiwa CD, Amuda MO, Afolabi SO, Ayotunde BT, Adejumo AA, Akindele AFI, Oyinloye BE, Owolabi OV, Genovese C, Ojo OA. Ajiboye BO, et al. Biochem Biophys Rep. 2024 May 16;38:101735. doi: 10.1016/j.bbrep.2024.101735. eCollection 2024 Jul. Biochem Biophys Rep. 2024. PMID: 38799115 Free PMC article. - A new look at Hsp70 activity in phosphatidylserine-enriched membranes: chaperone-induced quasi-interdigitated lipid phase.
Tagaeva R, Efimova S, Ischenko A, Zhakhov A, Shevtsov M, Ostroumova O. Tagaeva R, et al. Sci Rep. 2023 Nov 6;13(1):19233. doi: 10.1038/s41598-023-46131-x. Sci Rep. 2023. PMID: 37932471 Free PMC article. - A Comparative Study about the Neuroprotective Effects of DHA-Enriched Phosphatidylserine and EPA-Enriched Phosphatidylserine against Oxidative Damage in Primary Hippocampal Neurons.
Wang YW, Li Q, Li XY, Zhao YC, Wang CC, Xue CH, Wang YM, Zhang TT. Wang YW, et al. Mar Drugs. 2023 Jul 19;21(7):410. doi: 10.3390/md21070410. Mar Drugs. 2023. PMID: 37504941 Free PMC article. - Sevoflurane Exposure of Clinical Doses in Pregnant Rats Induces Vcan Changes without Significant Neural Apoptosis in the Offspring.
Jin Y, Hu X, Meng F, Luo Q, Liu H, Yang Z. Jin Y, et al. Medicina (Kaunas). 2023 Jan 17;59(2):190. doi: 10.3390/medicina59020190. Medicina (Kaunas). 2023. PMID: 36837392 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous