P granules extend the nuclear pore complex environment in the C. elegans germ line - PubMed (original) (raw)
P granules extend the nuclear pore complex environment in the C. elegans germ line
Dustin L Updike et al. J Cell Biol. 2011.
Abstract
The immortal and totipotent properties of the germ line depend on determinants within the germ plasm. A common characteristic of germ plasm across phyla is the presence of germ granules, including P granules in Caenorhabditis elegans, which are typically associated with the nuclear periphery. In C. elegans, nuclear pore complex (NPC)-like FG repeat domains are found in the VASA-related P-granule proteins GLH-1, GLH-2, and GLH-4 and other P-granule components. We demonstrate that P granules, like NPCs, are held together by weak hydrophobic interactions and establish a size-exclusion barrier. Our analysis of intestine-expressed proteins revealed that GLH-1 and its FG domain are not sufficient to form granules, but require factors like PGL-1 to nucleate the localized concentration of GLH proteins. GLH-1 is necessary but not sufficient for the perinuclear location of granules in the intestine. Our results suggest that P granules extend the NPC environment in the germ line and provide insights into the roles of the PGL and GLH family proteins.
Figures
Figure 1.
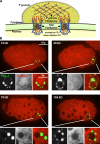
P granules create a size-exclusion barrier. (A) Model illustrating how P granules could extend the NPC environment through FG interactions. (B) C. elegans embryos from GFP::PGL-1 (green) worms injected with TRITC-labeled dextrans of different molecular weights (red).
Figure 2.
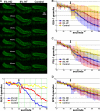
Hydrophobic interactions are required for P-granule integrity. (A) Top, dissected GLH-1::GFP gonads exposed to hexanediol (HD), hexanetriol (HT), or egg buffer (Control). Bottom, normalized P-granule counts from the image series in A. Arrow indicates the time HD, HT, or control buffer was added. See
Video 1
for complete time series. (B–D) GLH-1::GFP granule averages (B), GFP::PGL-1 granule averages (C), and GFP::SPD-2 granule averages (D) for HD, HT, and control. Standard deviations for each condition are shown in blue, red, and yellow shaded bars. 20 dissected gonads were analyzed for each treatment of each GFP line.
Figure 3.
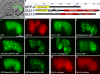
Ectopic expression of P-granule components in the intestine. (A) Comma-stage embryo expressing GFP behind the elt-2 intestinal promoter. (B) FG repeats (blue triangles), FG domains (yellow), zinc fingers (gray), and DEAD-box helicase domains (red) in NPP-4, GLH-1, and GLH-3. (C–N) 10-µm projections of different ectopic expression constructs in the intestine of comma-stage embryos.
Figure 4.
PGL-1 nucleates granule formation. (A) GFP-tagged and untagged PGL-1 expressed in the intestine. (B and D) GLH-1::mCherry (B) or GLH-3::mCherry (D) coexpressed with PGL-1::GFP in intestinal cells. (C and E) Young larvae expressing GLH-1::GFP (C) or the FG domain of GLH-1 tagged with GFP (E) in intestinal cells. The larvae In the right panels additionally express untagged PGL-1 in intestinal cells (transgenic PGL-1–expressing worms identified by myo-3::mCherry expression in body muscle).
Figure 5.
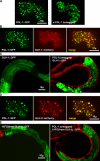
GLH-1 is necessary but not sufficient for the perinuclear association of PGL granules. (A–D) Single XY plane of comma-stage embryos expressing tagged PGL-1 or GLH-1 constructs, immunostained for nuclear pores (red) and either PGL-1 or GLH-1 (green), as noted. (A) Embryo expressing PGL-1 in the intestine. Ectopic PGL-1 granules in the intestine (arrowhead), endogenous PGL-1 granules in the germ cells (arrow). (B) Embryo expressing 3X(FG)-GLH-1 in the intestine. Cytoplasmic granules (arrowhead), perinuclear granules (arrow). (C) hpl-2(tm1489) embryo expressing GLH-1 in the intestine. Cytoplasmic granules (arrowhead), perinuclear granules (arrow). (D) GLH-1 (left) and 3X(FG)-GLH-1 (middle) coexpressed with PGL-1 in intestinal cells. 3XFG from GLH-1 fused to PGL-1 (right). Ectopic PGL-1 granules in the intestine (arrowhead), endogenous PGL-1 granules in the germ cells (arrow). (E) Wild-type and hpl-2(tm1489) L1s grown at 26°C on control bacteria and glh-1(RNAi) bacteria and immunostained for GLH-1 and PGL-3. Germ cells (arrowheads). (F) Germ lines from wild-type worms grown from L1 to adulthood at 26°C on control bacteria and glh-1(RNAi) bacteria.
Similar articles
- Germline Maintenance Through the Multifaceted Activities of GLH/Vasa in Caenorhabditis elegans P Granules.
Marnik EA, Fuqua JH, Sharp CS, Rochester JD, Xu EL, Holbrook SE, Updike DL. Marnik EA, et al. Genetics. 2019 Nov;213(3):923-939. doi: 10.1534/genetics.119.302670. Epub 2019 Sep 10. Genetics. 2019. PMID: 31506335 Free PMC article. - Genetic analysis of the Caenorhabditis elegans GLH family of P-granule proteins.
Spike C, Meyer N, Racen E, Orsborn A, Kirchner J, Kuznicki K, Yee C, Bennett K, Strome S. Spike C, et al. Genetics. 2008 Apr;178(4):1973-87. doi: 10.1534/genetics.107.083469. Genetics. 2008. PMID: 18430929 Free PMC article. - PGL proteins self associate and bind RNPs to mediate germ granule assembly in C. elegans.
Hanazawa M, Yonetani M, Sugimoto A. Hanazawa M, et al. J Cell Biol. 2011 Mar 21;192(6):929-37. doi: 10.1083/jcb.201010106. Epub 2011 Mar 14. J Cell Biol. 2011. PMID: 21402787 Free PMC article. - Connecting the Dots: Linking Caenorhabditis elegans Small RNA Pathways and Germ Granules.
Sundby AE, Molnar RI, Claycomb JM. Sundby AE, et al. Trends Cell Biol. 2021 May;31(5):387-401. doi: 10.1016/j.tcb.2020.12.012. Epub 2021 Jan 29. Trends Cell Biol. 2021. PMID: 33526340 Review. - Germ granules and gene regulation in the Caenorhabditis elegans germline.
Phillips CM, Updike DL. Phillips CM, et al. Genetics. 2022 Mar 3;220(3):iyab195. doi: 10.1093/genetics/iyab195. Genetics. 2022. PMID: 35239965 Free PMC article. Review.
Cited by
- PRMT5 and the role of symmetrical dimethylarginine in chromatoid bodies of planarian stem cells.
Rouhana L, Vieira AP, Roberts-Galbraith RH, Newmark PA. Rouhana L, et al. Development. 2012 Mar;139(6):1083-94. doi: 10.1242/dev.076182. Epub 2012 Feb 8. Development. 2012. PMID: 22318224 Free PMC article. - Phase transitions and size scaling of membrane-less organelles.
Brangwynne CP. Brangwynne CP. J Cell Biol. 2013 Dec 23;203(6):875-81. doi: 10.1083/jcb.201308087. J Cell Biol. 2013. PMID: 24368804 Free PMC article. Review. - RNA Helicase Vasa as a Multifunctional Conservative Regulator of Gametogenesis in Eukaryotes.
Adashev VE, Kotov AA, Olenina LV. Adashev VE, et al. Curr Issues Mol Biol. 2023 Jul 5;45(7):5677-5705. doi: 10.3390/cimb45070358. Curr Issues Mol Biol. 2023. PMID: 37504274 Free PMC article. Review. - Cell Biology of the Caenorhabditis elegans Nucleus.
Cohen-Fix O, Askjaer P. Cohen-Fix O, et al. Genetics. 2017 Jan;205(1):25-59. doi: 10.1534/genetics.116.197160. Genetics. 2017. PMID: 28049702 Free PMC article. Review. - Germ Granules Allow Transmission of Small RNA-Based Parental Responses in the "Germ Plasm".
Lev I, Rechavi O. Lev I, et al. iScience. 2020 Nov 19;23(12):101831. doi: 10.1016/j.isci.2020.101831. eCollection 2020 Dec 18. iScience. 2020. PMID: 33305186 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials