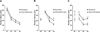A longitudinal study of behavioral deficits in an AβPP transgenic mouse model of Alzheimer's disease - PubMed (original) (raw)
A longitudinal study of behavioral deficits in an AβPP transgenic mouse model of Alzheimer's disease
Daniel Havas et al. J Alzheimers Dis. 2011.
Abstract
Elucidating the age-dependent alterations in transgenic (Tg) mice overexpressing amyloid-β protein precursor (AβPP) is important for understanding the pathogenesis of Alzheimer's disease (AD) and designing experimental therapies. Cross-studies have previously characterized some time-dependent behavioral and pathological alterations in AβPP Tg mice, however, a more comprehensive longitudinal study is needed to fully examine the progressive nature of behavioral deficits in these mice. In order to better understand the age- and gender-dependent progression of behavioral alterations, we performed a longitudinal study wherein Tg mice overexpressing human AβPP751 with the London (V717I) and Swedish (K670M/N671L) mutations under the regulatory control of the neuron specific murine (m)Thy-1 promoter (mThy1-hAβPP751) were behaviorally analyzed at 3 months and then re-tested at 6 and 9 months of age. The results show that there was an age-associated impairment in learning in the water maze task and habituation in the hole-board task. Motor coordination of the mThy1-hAβPP751 Tg mice was well-preserved throughout the investigated life span however, gender-specific deficits were observed in spontaneous activity and thigmotaxis. Neuropathologically, mThy1-hAβPP751 Tg mice displayed a progressive increase in the number of Aβ plaques and mean plaque size in the cortex and hippocampus from 3 to 6 and from 6 to 9 months of age. Taken together, these results indicate that the mThy1-hAβPP751 Tg mice model AD from the early onset of the disease through to later stages, allowing them to be utilized at numerous points during the timeline for drug test designs.
Figures
Figure 1. mThy1-hAβPP751 Tg mice display an age-related longitudinal decline in spatial memory and learning in the water maze
A) Learning curves in the non-Tg and mThy1-hAβPP751 Tg mice at 3 months of age in the water-maze over a four-day test period (D1 to D4). B) Learning curves in the non-Tg and mThy1-hAβPP751 Tg mice at 6 months of age in the water-maze over a four-day test period consisting of a retesting day (RT, where the platform was in the same position as on the last day of testing at 3 months of age) and three days during which the location of the platform was changed from trial to trial (D1 to D3). C) Learning curves in the non-Tg and mThy1-hAβPP751 Tg mice at 9 months of age in the water-maze over a four-day test period consisting of a retesting day (RT, where the platform was in the same position as on the last day of testing at 6 months of age) and three days during which the location of the platform was changed from trial to trial (D1 to D3). Data are represented by means (over four trials, each day) ± SEM. * Indicates significant difference between non-Tg and mThy1-hAβPP751 Tg mice with p<0.05 by one-way ANOVA; ** Indicates significant difference between non-Tg and mThy1-hAβPP751 Tg mice with p<0.01 by one-way ANOVA.
Figure 2. mThy1-hAβPP751 Tg mice display an age-related longitudinal decline memory retention in the water maze
A) Relearning ability of non-Tg and mThy1-hAβPP751 Tg mice at 6 months of age on the retest days (four trials in which the platform was in the same position it had been on the final day of testing at 3 months). B) Relearning ability of non-Tg and mThy1-hAβPP751 Tg mice at 9 months of age on the retest days (four trials in which the platform was in the same position it had been on the final day of testing at 6 months). C) Retention abilities of non-Tg and mThy1-hAβPP751 Tg mice in the water maze indicated by the number of target crossing in the probe trials. Data are represented by means ± SEM. * Indicates significant difference between female and male mThy1-hAβPP751 Tg mice with p<0.05 by one-way ANOVA; ** Indicates significant difference between non-Tg and mThy1-hAβPP751 Tg mice with p<0.01 by one-way ANOVA.
Figure 3. mThy1-hAβPP751 Tg mice display a curiosity deficit in the hole-board task
A) Curiosity levels of non-Tg and mThy1-hAβPP751 Tg mice expressed by the number beam breaks by nose or paw poking into the holes during a ten-minutes trial, at 3, 6, and 9 months of age. B) Gender stratification of the mThy1-hAβPP751 Tg mice at 3, 6, and 9 months of age. Data are represented by means ± SEM. * Indicates a significant difference between non-Tg and mThy1-hAβPP751 Tg mice with p<0.05 by one-way ANOVA ** Indicates a significant difference between non-Tg and mThy1-hAβPP751 Tg mice with p<0.01 by one-way ANOVA.
Figure 4. mThy1-hAβPP751 Tg mice display preserved motor function in the Open Field
A) Total activity in the Open Field of the non-Tg and mThy1-hAβPP751 Tg mice at 3, 6, and 9 months of age. B) Gender-stratification of the total activity in the mThy1-hAβPP751 Tg mice at 3, 6, and 9 months of age. C) Thigmotactic behavior in the non-Tg and mThy1-hAβPP751 Tg mice at 3, 6, and 9 months of age. D) Gender-stratification of thigmotaxis in the mThy1-hAβPP751 Tg mice at 3, 6, and 9 months of age. Data are presented as means ± SEM. * Indicates a significant difference between 3 and 6 month old non-Tg or 3 and 6 month old mThy1-hAβPP751 Tg mice with p<0.05 by one-way ANOVA. ** Indicates a significant difference between non-Tg and mThy1-hAβPP751 Tg mice with p<0.01 by one-way ANOVA.
Figure 5. mThy1-hAβPP751 Tg mice display preserved motor function in the Rotarod
Motor coordination in the non-Tg and mThy1-hAβPP751 Tg mice on the Rotorod, expressed as the revolutions per minute (rpm) at which mice were unable to remain on the rotating rod. Data are presented as means ± SEM.
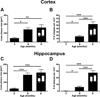
Figure 6. mThy1-hAβPP751 Tg mice display age-related, longitudinal accumulation of Aβ in the cortex and hippocampus
Aβ plaques were visualized using the 6E10 antibody to AA1-17 of the hAβ protein. A) Plaque size at 3, 6, and 9 months of age in the cortex of mThy1-hAβPP751 Tg mice. B) Plaque number at 3, 6, and 9 months of age in the cortex of mThy1-hAβPP751 Tg mice. C) Plaque size at 3, 6, and 9 months of age in the hippocampus of mThy1-hAβPP751 Tg mice. D) Plaque number at 3, 6, and 9 months of age in the hippocampus of mThy1-hAβPP751 Tg mice. Insets in the bars at 9 months indicate gender-related differences in cortical and hippocampal plaque size and number. Data are presented as means ± SEM.* Indicates a significant difference with p<0.05 by one-way ANOVA. ** Indicates a significant difference with p<0.01 by one-way ANOVA. *** Indicates a significant difference with p<0.001 by one-way ANOVA
Figure 7. Plasma and brain Aβ1–42 levels in mThy1-hAβPP751 Tg mice with age by ELISA
A) Plasma levels of soluble Aβ1–42 in the mThy1-hAβPP751 Tg mice at 3, 6, and 9 months of age. B) Insoluble hAβ1–42 in the FA fraction from the brain of mThy1-hAβPP751 Tg mice at 3, 6, and 9 months of age. Inset in the bar at 9 months indicates a gender-specific difference in FA- soluble hAβ1–42 at this age. Data are represented as means ± SEM * Indicates a significant difference with p<0.05 by one-way ANOVA. ** Indicates a significant difference with p<0.01 by one -way ANOVA. *** Indicates a significant difference with p<0.001 by one-way ANOVA.
Similar articles
- Insulin-resistant brain state after intracerebroventricular streptozotocin injection exacerbates Alzheimer-like changes in Tg2576 AbetaPP-overexpressing mice.
Plaschke K, Kopitz J, Siegelin M, Schliebs R, Salkovic-Petrisic M, Riederer P, Hoyer S. Plaschke K, et al. J Alzheimers Dis. 2010;19(2):691-704. doi: 10.3233/JAD-2010-1270. J Alzheimers Dis. 2010. PMID: 20110613 - Deletion of the cathepsin B gene improves memory deficits in a transgenic ALZHeimer's disease mouse model expressing AβPP containing the wild-type β-secretase site sequence.
Kindy MS, Yu J, Zhu H, El-Amouri SS, Hook V, Hook GR. Kindy MS, et al. J Alzheimers Dis. 2012;29(4):827-40. doi: 10.3233/JAD-2012-111604. J Alzheimers Dis. 2012. PMID: 22337825 Free PMC article. - Overexpression of Ubiquilin-1 Alleviates Alzheimer's Disease-Caused Cognitive and Motor Deficits and Reduces Amyloid-β Accumulation in Mice.
Adegoke OO, Qiao F, Liu Y, Longley K, Feng S, Wang H. Adegoke OO, et al. J Alzheimers Dis. 2017;59(2):575-590. doi: 10.3233/JAD-170173. J Alzheimers Dis. 2017. PMID: 28598849 Free PMC article. - Coenzyme Q10 decreases amyloid pathology and improves behavior in a transgenic mouse model of Alzheimer's disease.
Dumont M, Kipiani K, Yu F, Wille E, Katz M, Calingasan NY, Gouras GK, Lin MT, Beal MF. Dumont M, et al. J Alzheimers Dis. 2011;27(1):211-23. doi: 10.3233/JAD-2011-110209. J Alzheimers Dis. 2011. PMID: 21799249 Free PMC article. - CaV1.2 calcium channel expression in reactive astrocytes is associated with the formation of amyloid-β plaques in an Alzheimer's disease mouse model.
Daschil N, Obermair GJ, Flucher BE, Stefanova N, Hutter-Paier B, Windisch M, Humpel C, Marksteiner J. Daschil N, et al. J Alzheimers Dis. 2013;37(2):439-51. doi: 10.3233/JAD-130560. J Alzheimers Dis. 2013. PMID: 23948887 Free PMC article.
Cited by
- Thy1-hAPP(Lond/Swe+) mouse model of Alzheimer's disease displays broad behavioral deficits in sensorimotor, cognitive and social function.
Faizi M, Bader PL, Saw N, Nguyen TV, Beraki S, Wyss-Coray T, Longo FM, Shamloo M. Faizi M, et al. Brain Behav. 2012 Mar;2(2):142-54. doi: 10.1002/brb3.41. Brain Behav. 2012. PMID: 22574282 Free PMC article. - Reducing Endogenous α-Synuclein Mitigates the Degeneration of Selective Neuronal Populations in an Alzheimer's Disease Transgenic Mouse Model.
Spencer B, Desplats PA, Overk CR, Valera-Martin E, Rissman RA, Wu C, Mante M, Adame A, Florio J, Rockenstein E, Masliah E. Spencer B, et al. J Neurosci. 2016 Jul 27;36(30):7971-84. doi: 10.1523/JNEUROSCI.0775-16.2016. J Neurosci. 2016. PMID: 27466341 Free PMC article. - Hippocampal Reduction of α-Synuclein via RNA Interference Improves Neuropathology in Alzheimer's Disease Mice.
Leitão ADG, Spencer B, Sarsoza F, Ngolab J, Amalraj J, Masliah E, Wu C, Rissman RA. Leitão ADG, et al. J Alzheimers Dis. 2023;95(1):349-361. doi: 10.3233/JAD-230232. J Alzheimers Dis. 2023. PMID: 37522208 Free PMC article. - Neuroinflammation and related neuropathologies in APPSL mice: further value of this in vivo model of Alzheimer's disease.
Löffler T, Flunkert S, Havas D, Schweinzer C, Uger M, Windisch M, Steyrer E, Hutter-Paier B. Löffler T, et al. J Neuroinflammation. 2014 May 1;11:84. doi: 10.1186/1742-2094-11-84. J Neuroinflammation. 2014. PMID: 24886182 Free PMC article. - Small molecule p75NTR ligands reduce pathological phosphorylation and misfolding of tau, inflammatory changes, cholinergic degeneration, and cognitive deficits in AβPP(L/S) transgenic mice.
Nguyen TV, Shen L, Vander Griend L, Quach LN, Belichenko NP, Saw N, Yang T, Shamloo M, Wyss-Coray T, Massa SM, Longo FM. Nguyen TV, et al. J Alzheimers Dis. 2014;42(2):459-83. doi: 10.3233/JAD-140036. J Alzheimers Dis. 2014. PMID: 24898660 Free PMC article.
References
- Selkoe D. Amyloid β protein precursor and the pathogenesis of Alzheimer's disease. Cell. 1989;58:611–612. - PubMed
- Selkoe D. Amyloid β-protein deposition as a seminal pathogenic event in AD: an hypothesis. Neurobiol. Aging. 1990;11:299.
- Selkoe D. Physiological production of the β-amyloid protein and the mechanisms of Alzheimer's disease. Trends Neurosci. 1993;16:403–409. - PubMed
- Mandelkow E, Mandelkow E. Tau in Alzheimer's disease. Trends Cell. Biol. 1998;8:125–127. - PubMed
- Trojanowski JQ, Shin RW, Schmidt ML, Lee VM. Relationship between plaques, tangles, and dystrophic processes in Alzheimer's disease. Neurobiol Aging. 1995;16:335–340. discussion 341–335. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AG03197/AG/NIA NIH HHS/United States
- AG010435/AG/NIA NIH HHS/United States
- R01 AG018440/AG/NIA NIH HHS/United States
- AG022074/AG/NIA NIH HHS/United States
- R37 AG018440/AG/NIA NIH HHS/United States
- P01 AG010435/AG/NIA NIH HHS/United States
- AG18440/AG/NIA NIH HHS/United States
- P01 AG022074/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous