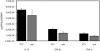Anti-inflammatory effects of FTY720 do not prevent neuronal cell loss in a rat model of optic neuritis - PubMed (original) (raw)
Anti-inflammatory effects of FTY720 do not prevent neuronal cell loss in a rat model of optic neuritis
Christian R Rau et al. Am J Pathol. 2011 Apr.
Abstract
In multiple sclerosis, long-term disability is caused by axonal and neuronal damage. Established therapies target primarily the inflammatory component of the disease, but fail to prevent neurodegeneration. Fingolimod (codenamed FTY720) is an oral sphingosine 1-phosphate (S1P) receptor modulator with promising results in phase II trials in multiple sclerosis patients and is under further development as a novel treatment for multiple sclerosis. To evaluate whether FTY720 has neuroprotective properties, we tested this drug in a rat model of myelin oligodendrocyte glycoprotein-induced optic neuritis. FTY720 exerted significant anti-inflammatory effects during optic neuritis and reduced inflammation, demyelination, and axonal damage; however, FTY720 treatment did not prevent apoptosis of retinal ganglion cells (RGCs), the neurons that form the axons of the optic nerve. Consistent with this lack of effect on RGC survival, FTY720 treatment did not improve visual function, nor did it prevent apoptosis of RGCs in vitro. We observed a persistent activation of apoptotic signaling pathways in RGCs under FTY720 treatment, a possible underlying mechanism for the lack of neuroprotection in the presence of strong anti-inflammatory effects, Furthermore, FTY720 shifted the remaining inflammation in the optic nerve toward neurotoxicity by modest up-regulation of potential neurotoxic cytokines. We conclude that FTY720-induced anti-inflammation and axon protection did not of itself protect neurons from apoptotic cell death.
Copyright © 2011 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures
Figure 1
Clinical score. Daily oral application of FTY720 started at day 7 after immunization significantly suppresses incidence of MOG-EAE. Severity of disease was also substantially reduced. Mean clinical score under treatment with FTY720 during disease course was compared with vehicle-treated animals (n = 10 for each group); all animals are included, with or without clinical symptoms. The clinical score represents primarily spinal cord lesions; visual function is not included.
Figure 2
Visual evoked potentials (VEP) and RGC survival. Electrophysiological measurement of the visual system at day 28 after immunization showed no improvement in VEP recordings in FTY720-treated animals, compared with vehicle-treated controls. A–C: Representative pattern VEP recordings of the left eye and the right eye of a sham-immunized control animal (A), FTY720-treated animal (B), and a vehicle-treated animal (C). D: Mean VEP score was 3.8 in the control rats immunized with complete Freund's adjuvant (ctrl), 2.4 in the vehicle group, and 2.6 in the FTY720-treated group (n = 10 in each group; P = 0.91466 for FTY720- vs. vehicle-treated animals). E: Mean density of RGCs in sham-immunized control (ctrl), FTY72-treated, and vehicle-treated animals at 28 days after immunization. There was no significant difference in RGC survival between the FTY720 treatment and vehicle treatment groups (P = 0.71256).
Figure 3
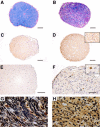
Optical nerve (ON) histopathology. A and B: Representative Luxol Fast Blue-stained cross-section of ONs of an FTY720-treated rat (A) shows only small areas of demyelination, with mainly intact myelin (blue); in contrast, a cross-section from a vehicle-treated control animal (B) shows extensively demyelinated areas (purple). C and D: Representative examples of the substantially higher number of ED1+ macrophages/activated microglia detected in the ON of a vehicle-treated control animal (D), compared with an animal that had received FTY720 (C). Inset: Higher-magnification view of ED1+ cells. E and F: Representative ON cross-sections showing reduced β-APP+ axons (arrows) under FTY720 treatment on EAE day 8 (E), compared with the vehicle-treated control (F). Appearance on day 28 after immunization was essentially the same as shown here (E) for day 8 of MOG-EAE. F, Inset: Higher-magnification view of a β-APP+ axon. G and H: Bielschowsky's silver impregnation of an ON cross-section reveals different densities of axons on EAE day 8 in FTY720-treated animals (G) and vehicle-treated control animals (H). Scale bars: 100 μm (A–F); 20 μm (G and H).
Figure 4
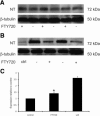
Nitrotyrosine (NT) expression. In Western blot analysisthe nitrotyrosine level in the retina (A) was unchanged, but in the optic nerve (B) the level was decreased in FTY720-treated animals (+), compared with vehicle-treated animals (−). β-Tubulin serves as a housekeeping protein. C: Quantitation of nitrotyrosine expression level in the optic nerve. *P < 0.05, compared with vehicle-treated animals.
Figure 5
Immunohistochemistry for the S1P1-receptor. In comparison of S1P1-receptor expression pattern between healthy and MOG-EAE animals, no expression was found in optic nerve (A) or retina (C) of healthy animals, but strong S1P1 expression was evident in diseased optic nerve (B), as well as in both healthy (D) and diseased (E) spinal cord. Scale bars: 100 μm (A–C); 20 μm (D and E). Insets: matching higher magnification view of S1P1-receptor-negative (A) and S1P1-receptor-positive (B, D and E) cells.
Figure 6
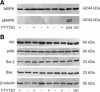
Western blot analysis. Western blots of retinal protein lysates obtained on the day of clinical manifestation of MOG-EAE revealed that FTY720 treatment (+) resulted in unchanged protein levels of Bcl-2 and Bax, compared with treatment with vehicle alone (−). Analyses of phosphorylation levels of Akt and MAPK 1/2 also revealed unchanged protein expression. β-Tubulin serves as a housekeeping protein.
Figure 7
Enzyme-linked immunosorbent assay analysis of the expression pattern of neuroprotective cytokines and neurotrophins in the ON under influence of FTY720. Levels of IL-12, TGF-β1, and TNF-α were similar in ON lysates of the FTY720- and vehicle-treated animals (n = 10 in each group).
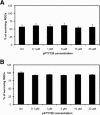
Figure 8
pFTY720 had no direct influence on RGC survival in vitro in cell culture with (A) or without (B) growth factor withdrawal. The numbers of surviving immunopurified RGCs treated with different concentrations of pFTY720 are expressed as a percentage of the matching control.
Similar articles
- FTY720 (fingolimod) efficacy in an animal model of multiple sclerosis requires astrocyte sphingosine 1-phosphate receptor 1 (S1P1) modulation.
Choi JW, Gardell SE, Herr DR, Rivera R, Lee CW, Noguchi K, Teo ST, Yung YC, Lu M, Kennedy G, Chun J. Choi JW, et al. Proc Natl Acad Sci U S A. 2011 Jan 11;108(2):751-6. doi: 10.1073/pnas.1014154108. Epub 2010 Dec 21. Proc Natl Acad Sci U S A. 2011. PMID: 21177428 Free PMC article. - FTY720 ameliorates MOG-induced experimental autoimmune encephalomyelitis by suppressing both cellular and humoral immune responses.
Papadopoulos D, Rundle J, Patel R, Marshall I, Stretton J, Eaton R, Richardson JC, Gonzalez MI, Philpott KL, Reynolds R. Papadopoulos D, et al. J Neurosci Res. 2010 Feb 1;88(2):346-59. doi: 10.1002/jnr.22196. J Neurosci Res. 2010. PMID: 19658199 - Sphingosine 1-phosphate receptor modulator fingolimod (FTY720) does not promote remyelination in vivo.
Hu Y, Lee X, Ji B, Guckian K, Apicco D, Pepinsky RB, Miller RH, Mi S. Hu Y, et al. Mol Cell Neurosci. 2011 Sep;48(1):72-81. doi: 10.1016/j.mcn.2011.06.007. Epub 2011 Jun 24. Mol Cell Neurosci. 2011. PMID: 21740973 - FTY720 (fingolimod) in Multiple Sclerosis: therapeutic effects in the immune and the central nervous system.
Brinkmann V. Brinkmann V. Br J Pharmacol. 2009 Nov;158(5):1173-82. doi: 10.1111/j.1476-5381.2009.00451.x. Epub 2009 Oct 8. Br J Pharmacol. 2009. PMID: 19814729 Free PMC article. Review. - Ascomycete derivative to MS therapeutic: S1P receptor modulator FTY720.
Hiestand PC, Rausch M, Meier DP, Foster CA. Hiestand PC, et al. Prog Drug Res. 2008;66:361, 363-81. doi: 10.1007/978-3-7643-8595-8_8. Prog Drug Res. 2008. PMID: 18416311 Review.
Cited by
- Diffusion tensor imaging detects treatment effects of FTY720 in experimental autoimmune encephalomyelitis mice.
Wang X, Brieland JK, Kim JH, Chen YJ, O'Neal J, O'Neil SP, Tu TW, Trinkaus K, Song SK. Wang X, et al. NMR Biomed. 2013 Dec;26(12):1742-50. doi: 10.1002/nbm.3012. Epub 2013 Aug 13. NMR Biomed. 2013. PMID: 23939596 Free PMC article. - Lysophospholipids and their receptors in the central nervous system.
Choi JW, Chun J. Choi JW, et al. Biochim Biophys Acta. 2013 Jan;1831(1):20-32. doi: 10.1016/j.bbalip.2012.07.015. Epub 2012 Jul 31. Biochim Biophys Acta. 2013. PMID: 22884303 Free PMC article. Review. - The functional antagonist of sphingosine-1-phosphate, FTY720, impairs gut barrier function.
Sikdar S, Mitra D, Das O, Bhaumik M, Dutta S. Sikdar S, et al. Front Pharmacol. 2024 Aug 19;15:1407228. doi: 10.3389/fphar.2024.1407228. eCollection 2024. Front Pharmacol. 2024. PMID: 39224783 Free PMC article. - Sphingolipids in ocular inflammation.
Chan AY, Mann SN, Chen H, Stone DU, Carr DJ, Mandal NA. Chan AY, et al. Adv Exp Med Biol. 2014;801:623-9. doi: 10.1007/978-1-4614-3209-8_78. Adv Exp Med Biol. 2014. PMID: 24664751 Free PMC article. Review. - Neurodegeneration in Autoimmune Optic Neuritis Is Associated with Altered APP Cleavage in Neurons and Up-Regulation of p53.
Herold S, Kumar P, Wichert SP, Kretzschmar B, Bähr M, Rossner MJ, Hein K. Herold S, et al. PLoS One. 2015 Oct 1;10(10):e0138852. doi: 10.1371/journal.pone.0138852. eCollection 2015. PLoS One. 2015. PMID: 26426258 Free PMC article.
References
- Trapp B.D., Peterson J., Ransohoff R.M., Rudick R., Mörk S., Bö L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338:278–285. - PubMed
- Kornek B., Storch M.K., Weissert R., Wallstroem E., Stefferl A., Olsson T., Linington C., Schmidbauer M., Lassmann H. Multiple sclerosis and chronic autoimmune encephalomyelitis: a comparative quantitative study of axonal injury in active, inactive, and remyelinated lesions. Am J Pathol. 2000;157:267–276. - PMC - PubMed
- Brex P.A., Ciccarelli O., O'Riordan J.I., Sailer M., Thompson A.J., Miller D.H. A longitudinal study of abnormalities on MRI and disability from multiple sclerosis. N Engl J Med. 2002;346:158–164. - PubMed
- Cohen J.A., Barkhof F., Comi G., Hartung H.P., Khatri B.O., Montalban X., Pelletier J., Capra R., Gallo P., Izquierdo G., Tiel-Wilck K., de Vera A., Jin J., Stites T., Wu S., Aradhye S., Kappos L; TRANSFORMS Study Group. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010;362:402–415. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources