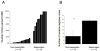Imaging dopamine transmission in cocaine dependence: link between neurochemistry and response to treatment - PubMed (original) (raw)
Imaging dopamine transmission in cocaine dependence: link between neurochemistry and response to treatment
Diana Martinez et al. Am J Psychiatry. 2011 Jun.
Erratum in
- Am J Psychiatry. 2011 May;168(5):553
Abstract
Objective: Previous research has shown that dopamine signaling in the limbic striatum is crucial for selecting adaptive, motivated behavior and that disrupted dopamine transmission is associated with impulsive and maladaptive behavior. In humans, positron emission tomography (PET) imaging studies have shown that cocaine dependence is associated with the dysregulation of striatal dopamine signaling, which is linked to cocaine-seeking behavior. The goal of the present study was to investigate whether this association applies to the treatment setting. The authors hypothesized that dopamine signaling in the limbic striatum would be associated with response to a behavioral treatment that uses positive reinforcement to replace impulsive cocaine use with constructive personal goals.
Method: Prior to treatment, cocaine-dependent subjects underwent two PET scans using [(11)C]raclopride, before and after the administration of a stimulant (methylphenidate), for measurement of striatal dopamine D(2/3) receptor binding and presynaptic dopamine release.
Results: Both of the outcome measures were lower in the volunteers who did not respond to treatment than in those who experienced a positive treatment response.
Conclusions: These findings provide insight into the neurochemistry of treatment response and show that low dopamine transmission is associated with treatment failure. In addition, these data suggest that the combination of behavioral treatment with methods that increase striatal dopamine signaling might serve as a therapeutic strategy for cocaine dependence.
Conflict of interest statement
Disclosure/Conflicts of Interest:
The authors report no conflicts of interest associated with the content of this manuscript.
Diana Martinez: no disclosures
Kenneth Carpentar: no disclosures
Fei Liu: no disclosures
Mark Slifstein: is a consultant for Glaxo-Smith-Kline and Amgen.
Allegra Broft: no disclosures
Alessandra Calvo-Friedman: no disclosures
Dileep Kumar: no disclosures
Ronald Van Heertum: no disclosures
Herbert D Kleber: is a consultant for Abbott Labs, The Grunenthal Group, Johnson & Johnson, Purdue Pharma, Reckett Benckiser, Alkermes Pharmaceuticals
Edward Nunes: no disclosures
Figures
Figure 1
A) Plot of all cocaine dependent subjects (n = 25) showing the amount of voucher money earned for cocaine-negative urine samples (range 0–0 – 0–977.50). The subjects’ response to treatment shows a bimodal distribution, which was used to classify subjects as responders or non-responders. B) Comparison of the average number of cocaine-negative urines provided over 12 weeks in the non-responders and responders (range 0–36). The values are the average and standard deviation for each group.
Figure 2
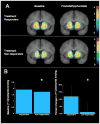
A) Average [11C]raclopride D2/3 receptor binding (BPND) in the treatment responders (top) and non-responders (bottom). The scans shown are before (left) and after (right) 60 mg PO methylphenidate administration, which increases extracellular dopamine so that fewer D2/3 receptors are available to bind to [11C]raclopride. The color bar shows the values for BPND. B) Bar graphs showing the differences between the treatment responders and non-responders in the limbic striatum for (left) BPND (pre-methylphenidate D2/3 receptor binding) and (right) ΔBPND, the percent decrease in methylphenidate-induced [11C]raclopride binding. These data show that treatment responders had higher dopamine D2/3 receptor binding and greater pre-synaptic dopamine release compared to non-responders in the limbic striatum.
Comment in
- Cracking the code: dopamine signaling in cocaine dependence.
Martis B. Martis B. Am J Psychiatry. 2011 Jun;168(6):572-5. doi: 10.1176/appi.ajp.2011.11030470. Am J Psychiatry. 2011. PMID: 21642479 No abstract available.
Similar articles
- Lower level of endogenous dopamine in patients with cocaine dependence: findings from PET imaging of D(2)/D(3) receptors following acute dopamine depletion.
Martinez D, Greene K, Broft A, Kumar D, Liu F, Narendran R, Slifstein M, Van Heertum R, Kleber HD. Martinez D, et al. Am J Psychiatry. 2009 Oct;166(10):1170-7. doi: 10.1176/appi.ajp.2009.08121801. Epub 2009 Sep 1. Am J Psychiatry. 2009. PMID: 19723785 Free PMC article. - Cocaine dependence and d2 receptor availability in the functional subdivisions of the striatum: relationship with cocaine-seeking behavior.
Martinez D, Broft A, Foltin RW, Slifstein M, Hwang DR, Huang Y, Perez A, Frankle WG, Cooper T, Kleber HD, Fischman MW, Laruelle M. Martinez D, et al. Neuropsychopharmacology. 2004 Jun;29(6):1190-202. doi: 10.1038/sj.npp.1300420. Neuropsychopharmacology. 2004. PMID: 15010698 - Deficits in dopamine D(2) receptors and presynaptic dopamine in heroin dependence: commonalities and differences with other types of addiction.
Martinez D, Saccone PA, Liu F, Slifstein M, Orlowska D, Grassetti A, Cook S, Broft A, Van Heertum R, Comer SD. Martinez D, et al. Biol Psychiatry. 2012 Feb 1;71(3):192-8. doi: 10.1016/j.biopsych.2011.08.024. Epub 2011 Oct 19. Biol Psychiatry. 2012. PMID: 22015315 Free PMC article. Clinical Trial. - Association of Stimulant Use With Dopaminergic Alterations in Users of Cocaine, Amphetamine, or Methamphetamine: A Systematic Review and Meta-analysis.
Ashok AH, Mizuno Y, Volkow ND, Howes OD. Ashok AH, et al. JAMA Psychiatry. 2017 May 1;74(5):511-519. doi: 10.1001/jamapsychiatry.2017.0135. JAMA Psychiatry. 2017. PMID: 28297025 Free PMC article. Review. - PET imaging of dopamine D2 receptors in monkey models of cocaine abuse: genetic predisposition versus environmental modulation.
Nader MA, Czoty PW. Nader MA, et al. Am J Psychiatry. 2005 Aug;162(8):1473-82. doi: 10.1176/appi.ajp.162.8.1473. Am J Psychiatry. 2005. PMID: 16055768 Review.
Cited by
- Imaging addiction: D2 receptors and dopamine signaling in the striatum as biomarkers for impulsivity.
Trifilieff P, Martinez D. Trifilieff P, et al. Neuropharmacology. 2014 Jan;76 Pt B(0 0):498-509. doi: 10.1016/j.neuropharm.2013.06.031. Epub 2013 Jul 10. Neuropharmacology. 2014. PMID: 23851257 Free PMC article. Review. - Anhedonia Is Associated with Poorer Outcomes in Contingency Management for Cocaine Use Disorder.
Wardle MC, Vincent JN, Suchting R, Green CE, Lane SD, Schmitz JM. Wardle MC, et al. J Subst Abuse Treat. 2017 Jan;72:32-39. doi: 10.1016/j.jsat.2016.08.020. Epub 2016 Sep 9. J Subst Abuse Treat. 2017. PMID: 27646197 Free PMC article. Clinical Trial. - Higher binding of the dopamine D3 receptor-preferring ligand [11C]-(+)-propyl-hexahydro-naphtho-oxazin in methamphetamine polydrug users: a positron emission tomography study.
Boileau I, Payer D, Houle S, Behzadi A, Rusjan PM, Tong J, Wilkins D, Selby P, George TP, Zack M, Furukawa Y, McCluskey T, Wilson AA, Kish SJ. Boileau I, et al. J Neurosci. 2012 Jan 25;32(4):1353-9. doi: 10.1523/JNEUROSCI.4371-11.2012. J Neurosci. 2012. PMID: 22279219 Free PMC article. - Imaging phosphodiesterase-10a availability in cocaine use disorder with [11 C]IMA107 and PET.
Tollefson S, Gertler J, Himes ML, Paris J, Kendro S, Lopresti B, Scott Mason N, Narendran R. Tollefson S, et al. Synapse. 2019 Jan;73(1):e22070. doi: 10.1002/syn.22070. Epub 2018 Sep 27. Synapse. 2019. PMID: 30240027 Free PMC article. - Mixed amphetamine salts-extended release (MAS-ER) as a behavioral treatment augmentation strategy for cocaine use disorder: A randomized clinical trial.
Carpenter KM, Choi CJ, Basaraba C, Pavlicova M, Brooks DJ, Brezing CA, Bisaga A, Nunes EV, Mariani JJ, Levin FR. Carpenter KM, et al. Exp Clin Psychopharmacol. 2024 Feb;32(1):112-127. doi: 10.1037/pha0000676. Epub 2023 Sep 21. Exp Clin Psychopharmacol. 2024. PMID: 37732961 Free PMC article. Clinical Trial.
References
- Poling J, Kosten TR, Sofuoglu M. Treatment outcome predictors for cocaine dependence. Am J Drug Alcohol Abuse. 2007;33(2):191–206. - PubMed
- Wise RA. Addictive drugs and brain stimulation reward. Annu Rev Neurosci. 1996;19:319–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K02 DA-026525/DA/NIDA NIH HHS/United States
- R01 DA020855/DA/NIDA NIH HHS/United States
- UL1 RR024156/RR/NCRR NIH HHS/United States
- K24 DA-022412/DA/NIDA NIH HHS/United States
- R01 DA-020855/DA/NIDA NIH HHS/United States
- R01 DA020855-02/DA/NIDA NIH HHS/United States
- R01 DA020855-01/DA/NIDA NIH HHS/United States
- K02 DA026525/DA/NIDA NIH HHS/United States
- R01 DA020855-03/DA/NIDA NIH HHS/United States
- R01 DA020855-04/DA/NIDA NIH HHS/United States
- K24 DA022412/DA/NIDA NIH HHS/United States
- UL1 RR-024156-03/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical