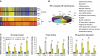The unfolded protein response transducer IRE1α prevents ER stress-induced hepatic steatosis - PubMed (original) (raw)
. 2011 Apr 6;30(7):1357-75.
doi: 10.1038/emboj.2011.52. Epub 2011 Mar 15.
Shiyu Wang, Jyoti Malhotra, Justin R Hassler, Sung Hoon Back, Guohui Wang, Lin Chang, Wenbo Xu, Hongzhi Miao, Roberta Leonardi, Y Eugene Chen, Suzanne Jackowski, Randal J Kaufman
Affiliations
- PMID: 21407177
- PMCID: PMC3094110
- DOI: 10.1038/emboj.2011.52
The unfolded protein response transducer IRE1α prevents ER stress-induced hepatic steatosis
Kezhong Zhang et al. EMBO J. 2011.
Abstract
The endoplasmic reticulum (ER) is the cellular organelle responsible for protein folding and assembly, lipid and sterol biosynthesis, and calcium storage. The unfolded protein response (UPR) is an adaptive intracellular stress response to accumulation of unfolded or misfolded proteins in the ER. In this study, we show that the most conserved UPR sensor inositol-requiring enzyme 1 α (IRE1α), an ER transmembrane protein kinase/endoribonuclease, is required to maintain hepatic lipid homeostasis under ER stress conditions through repressing hepatic lipid accumulation and maintaining lipoprotein secretion. To elucidate physiological roles of IRE1α-mediated signalling in the liver, we generated hepatocyte-specific Ire1α-null mice by utilizing an albumin promoter-controlled Cre recombinase-mediated deletion. Deletion of Ire1α caused defective induction of genes encoding functions in ER-to-Golgi protein transport, oxidative protein folding, and ER-associated degradation (ERAD) of misfolded proteins, and led to selective induction of pro-apoptotic UPR trans-activators. We show that IRE1α is required to maintain the secretion efficiency of selective proteins. In the absence of ER stress, mice with hepatocyte-specific Ire1α deletion displayed modest hepatosteatosis that became profound after induction of ER stress. Further investigation revealed that IRE1α represses expression of key metabolic transcriptional regulators, including CCAAT/enhancer-binding protein (C/EBP) β, C/EBPδ, peroxisome proliferator-activated receptor γ (PPARγ), and enzymes involved in triglyceride biosynthesis. IRE1α was also found to be required for efficient secretion of apolipoproteins upon disruption of ER homeostasis. Consistent with a role for IRE1α in preventing intracellular lipid accumulation, mice with hepatocyte-specific deletion of Ire1α developed severe hepatic steatosis after treatment with an ER stress-inducing anti-cancer drug Bortezomib, upon expression of a misfolding-prone human blood clotting factor VIII, or after partial hepatectomy. The identification of IRE1α as a key regulator to prevent hepatic steatosis provides novel insights into ER stress mechanisms in fatty liver diseases associated with toxic liver injuries.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Figure 1
Hepatocyte-specific deletion of _Ire1_α. (A) Schematic illustration of the targeting vector and the _Ire1_α-null alleles. The targeting vector contained loxP-flanked exons 16 and 17 of the murine _Ire1_α gene as well as a loxP-flanked Neomycin (Neo) cassette. The engineered mice containing the targeted allele were backcrossed with ZP3-Cre transgenic mice in order to generate mice with the loxP-flanked _Ire1_α allele without the Neo cassette. This mouse strain was then crossed with heterozygous _Ire1_α-null mice (Lee et al, 2002; Zhang et al, 2005) to produce mice containing an _Ire1_α conditional allele (Ire1_α_fe) and an _Ire1_α-null allele (_Ire1_α−). (B) Genotyping of conditional _Ire1_α-null mice. Mice harbouring a conditional _Ire1_α allele in the presence of an _Ire1_α-null or wild-type allele were crossed with transgenic mice that express Cre recombinase under the control of the albumin promoter to generate hepatocyte-specific _Ire1_α-null mice (Ire1_α_Hepfe/−) and control mice that harbour a functional _Ire1_α allele (Ire1_α_Hepfe/+). Three pairs of PCR reactions were used for genotyping: one pair amplifies the targeted allele and the wild-type allele; a second pair amplifies the Neo cassette to identify the _Ire1_α-null allele (Lee et al, 2002; Zhang et al, 2005); and a third set amplifies the Cre transgene. (C) Semiquantitative reverse transcription (RT)–PCR of Xbp1 mRNA. Xbp1 mRNA splicing was not detected in livers of hepatocyte-specific _Ire1_α-null mice (Ire1_α_Hepfe/−) at 8 h after intraperitoneal injection of TM (2 μg/g body weight). (D, E) Liver sections from hepatocyte-specific _Ire1_α-null (Ire1_α_Hepfe/−) and control (Ire1_α_Hepfe/+) mice in the absence of TM challenge stained with haematoxylin and eosin (magnification × 200) (D) and transmission electron microscopy (magnification × 19 000) (E).
Figure 2
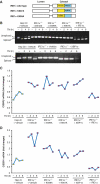
IRE1α regulates expression of genes involved in protein folding, ER-to-Golgi transport, and ERAD. (A) Affymetrix microarray analysis of mRNA expression profiles in livers of Ire1_α_Hepfe/− and Ire1_α_Hepfe/+ mice at 3 months of age at 8 h after intraperitoneal injection of TM (2 μg/g body weight) or vehicle (150 μM dextrose). Graphic representation of ANOVA analysis is shown for the expression of 177 genes (_P_-value cutoff was <0.05) that were significantly regulated upon TM injection into control mice (Ire1_α_Hepfe/+). Each vertical bar represents a single gene. Blue indicates lower expression and red indicates higher expression. (B) Percentages of IRE1α-regulated TM-inducible genes. The 177 TM-regulated genes identified by ANOVA were clustered based on their functions. The percentages of biological pathway-specific gene groups that are regulated by IRE1α are shown. (C) Quantitative real-time RT–PCR analysis of liver mRNA isolated from Ire1_α_Hepfe/− and Ire1_α_Hepfe/+ mice. Total liver mRNA was isolated at 8 h after injection with vehicle or TM (2 μg/g body weight) for real-time RT–PCR analysis. Expression values were normalized to β-actin mRNA levels. Fold changes of mRNA are shown in TM-treated mice compared with control mice. Each bar denotes the mean±s.e.m. (_n_=6 mice per group); *P<0.05, **P<0.01. _P_-values are shown for statistically significant differences. Edem1, ER degradation enhancing, mannosidase α-like 1; Sec22l1, SEC22 vesicle trafficking protein-like 1; Sec61α1, Sec61 α 1 subunit; Sec24d, SEC24-related gene family, member D; Tmed3, transmembrane emp24 domain containing 3; Pdi4, protein disulfide isomerase associated 4; Fkbp11, FK506-binding protein 11 of the peptidyl-prolyl cis_–_trans isomerase family; ERo1α, ER oxidoreductase-1 α, ERo1lβ, ERO1-like β; Hrd1, or Syvn1, synovial apoptosis inhibitor 1; ERdj4, DnaJ (Hsp40) homologue, subfamily B, member 9; ERdj3, DnaJ (Hsp40) homologue, subfamily B, member 11; P58(IPK), or Dnajc3, DnaJ (Hsp40) homologue, subfamily C, member 3.
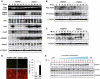
Figure 3
_Ire1_α deletion leads to upregulation of selective UPR genes in response to ER stress. (A–C) Xbp1 mRNA splicing and western blot analysis of livers from Ire1_α_Hepfe/− and Ire1_α_Hepfe/+ mice at 3 months of age after intraperitoneal injection of TM (2 μg/g body weight) for 8, 24, 36, 48, and 72 h. Mice injected with the vehicle (150 μM dextrose) were included as the control time point 0. (A) Semiquantitative RT–PCR analysis of Xbp1 mRNA splicing and western blot analysis of GRP94, GRP78/BiP, ATF4, ATF3, CHOP, GADD34, and caspase-3. Levels of α-tubulin were included as internal controls. The values below the gels represent protein signal intensities that were quantified using NIH ImageJ software and normalized to α-tubulin. (B, C) Western blot analysis of phosphorylated PERK, phosphorylated eIF2α, and ATF6. The values below the gels represent normalized protein signal intensities. (D) Immunofluorescence TUNEL staining of liver tissue sections for DNA fragmentation. Liver tissue sections were prepared from the Ire1_α_Hepfe/− and Ire1_α_Hepfe/+ mice after intraperitoneal injection of TM (2 μg/g body weight) for 36 h for TUNEL staining. The green fluorescence represents TUNEL-positive and red fluorescence represents propidium iodide (PI)-positive cells (magnification × 400). The right panel shows the percentages of TUNEL-positive cells determined by calculating the number of TUNEL-positive cells divided by the number of PI-positive cells from five fields of each slide. *P<0.05. (E) Western blot analysis of GRP94 and CHOP in immortalized Ire1_α-null (Ire1_α_fe/−_Cre) and control (Ire1_α_fe/fe) hepatocytes challenged with TM (2 μg/ml) for indicated times. _Ire1_α-null and control hepatocytes cultured in the absence of TM (at 0 and 24 h) were included as controls. Levels of GAPDH protein were determined as internal controls. The values below the gels represent protein signal intensities after normalization to GAPDH. C, control hepatocytes; K, _Ire1_α-null hepatocytes.
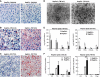
Figure 4
Hepatocyte-specific _Ire1_α deletion leads to hepatosteatosis and reduced plasma lipids. Ire1_α_Hepfe/− and Ire1_α_Hepfe/+ mice at 3 months of age were injected with TM (2 μg/g body weight) or vehicle (150 μM dextrose). At 8, 24, and 72 h after TM injection, liver tissues and plasma samples were isolated for lipid analysis. (A–C) Oil-red O staining of lipid droplets in the livers of Ire1_α_Hepfe/− and Ire1_α_Hepfe/+ mice after TM challenge for indicated time periods (magnification × 200). (D) Transmission electron micrographs of liver tissue sections from Ire1_α_Hepfe/− and Ire1_α_Hepfe/+ mice at 24 h after TM injection (magnification: × 3500). (E) Levels of plasma lipids in the Ire1_α_Hepfe/− and Ire1_α_Hepfe/+ mice at 8 h after injection with TM or vehicle. Chol, total plasma cholesterol; TG, triglycerides; HDL, high-density lipoproteins; (V) LDL, low and very low-density lipoproteins. Each bar denotes the mean±s.e.m. (_n_=6 mice per group); *P<0.05. _P_-values are shown for statistically significant differences. (F) Levels of liver cellular lipids in the Ire1_α_Hepfe/− and Ire1_α_Hepfe/+ mice at 8 h after injection with TM or vehicle. CE, cholesterol ester; TG, triglycerides; FA, fatty acids; Chol, total liver cholesterol. Each bar denotes the mean±s.e.m. (_n_=3 mice per group); *P<0.05.
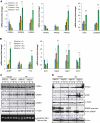
Figure 5
IRE1α represses expression of key lipogenic regulators in the liver in response to ER stress. (A, B) Quantitative real-time RT–PCR analysis of liver mRNA in Ire1_α_Hepfe/− and Ire1_α_Hepfe/+ mice. Total RNAs from the livers of Ire1_α_Hepfe/− and Ire1_α_Hepfe/+ mice at 3 months of age at 8 h after injection with TM (2 μg/g body weight) or vehicle (V) were subjected to quantitative real-time RT–PCR analysis. Expression values were normalized to β-actin mRNA. Fold changes are shown relative to mRNA expression in one of the control (Ire1_α_Hepfe/+) untreated mouse livers. Each bar denotes the mean±s.e.m. (_n_=6 mice per group); *P<0.05; **P<0.01. _P_-values are shown for statistically significant differences. (C, D) Western blot analysis of liver tissue from Ire1_α_Hepfe/− and Ire1_α_Hepfe/+ mice at 8 h after injection with TM (2 μg/g body weight) or vehicle. Semiquantitative RT–PCR analysis was performed to measure the levels of spliced and unspliced Xbp1 mRNAs as an indicator of IRE1α activity in the livers (C, bottom panel). In western blot analysis of C/EBPβ, we detected the C/EBPβ LAP (liver-enriched transcriptional activator protein) isoform of about 35 kDa in the mouse liver tissue samples (Calkhoven et al, 2000). Because PPARγ2 was not detected at the mRNA level in the liver tissue in the absence or presence of TM challenge (A, B), the PPARγ signals from the western blot analysis likely reflected PPARγ1. The experiments were repeated at least three times with consistent results, and representative data are shown. The values below the gels represent protein signal intensities after normalization to that of α-tubulin.
Figure 6
Overexpression of IRE1α suppresses upregulation of CEBPβ and CEBPδ in _Ire1_α-null hepatocytes. (A) Depiction of domain structures for wild-type and mutant versions of IRE1α protein. K599A, IRE1α kinase mutant; K907A, IRE1α RNase mutant. _Ire1_α-null or control hepatocytes were infected with recombinant adenoviruses expressing different versions of IRE1α, spliced Xbp1 mRNA, or empty vector control. The infected hepatocytes were then treated with TM (2 μg/ml) for the times indicated. (B) Semiquantitative RT–PCR analysis of spliced and unspliced Xbp1 mRNAs in the Ire1_α-null (Ire1_α_fe/−_CRE) or control (Ire1_α_fe/fe) hepatocytes. (C, D) Quantitative real-time RT–PCR analysis of _C/ebp_β and _C/ebp_δ mRNAs in adenovirus-infected hepatocytes. At 48 h after infection of _Ire1_α-null or control hepatocytes with the indicated adenoviruses, the hepatocytes were treated with TM (5 μg/ml) for the times indicated. Expression values of _C/ebp_β and _C/ebp_δ mRNAs were normalized to β-actin mRNA. Fold changes of mRNA were measured by comparing to the expression level of mRNA in one of the empty virus-transfected control cells. Hep ctl, control (Ire1_α_fe/fe) hepatocytes; _Ire1_α−/−, Ire1_α-null (Ire1_α_fe/−_CRE) hepatocytes. A full-colour version of this figure is available at The EMBO Journal Online.
Figure 7
IRE1α is required for efficient secretion of ApoB-containing apolipoproteins. (A, B) Western blot analysis of ApoB in the liver and blood plasma samples from _Ire1_α-null (Ire1_α_Hepfe/−) and control (Ire1_α_Hepfe/+) mice at 24 h after injection with TM (2 μg/g body weight) or vehicle. Levels of fatty acid synthase in the liver tissues and albumin in the plasma were measured as internal controls. The values below the gels represent the normalized protein signal intensities. FASN, fatty acid synthase; Alb, albumin. (C) Primary hepatocytes from _Ire1_α-null (Ire1_α_fe/−) and control (Ire1_α_fe/+) mice were labelled with [35S]methionine/cysteine for 25 min and then chased for the indicated times. For TM-treated samples, the primary hepatocytes were treated with TM (10 μg/ml) for 6 h before and throughout the pulse-chase experiments. Radiolabelled ApoB-containing apolipoproteins were immunoprecipitated from the cell lysates and the media with a rabbit anti-ApoB polyclonal antibody, and resolved by 5% SDS–polyacrylamide gel electrophoresis with fluorography. The experiments were repeated at least three times with consistent results, and representative data are shown. (D) Quantification of secretion rates of ApoB48- or ApoB100-containing apolipoproteins from _Ire1_α-null (Ire1_α_fe/−) or control (Ire1_α_fe/+) primary hepatocytes to culture media in the absence or presence of TM treatment, as indicated in the panel (C). Secretion rates of ApoB=secreted ApoB48(100)/cellular ApoB48(100). Levels of secreted ApoB48(100) were quantified based on the signals of ApoB48(100) present in the culture media, and levels of cellular ApoB48(100) were quantified based on the signals of ApoB48(100) present in the cellular lysates of the _Ire1_α-null or control primary hepatocytes, as shown in the panel (C). A full-colour version of this figure is available at The EMBO Journal Online.
Figure 8
Mice with hepatocyte-specific _Ire1_α deletion exhibit severe hepatic steatosis in response to Bortezomib treatment or expression of a misfolding-prone hFVIII. (A–C) _Ire1_α-null (Ire1_α_Hepfe/−) and control (Ire1_α_Hepfe/+) mice at 3 months of age were injected with Bortezomib (1 μg/g body weight) into the tail-vein. At 36 h after injection, the mice were fasted for 8 h prior to euthanasia and collection of samples. (A) Quantitative real-time RT–PCR analysis for expression of Chop and spliced Xbp1 mRNAs in the liver. Expression values were normalized to β-actin mRNA. Fold changes of mRNA levels were determined by comparison to the expression level in one of the controls. Each bar denotes the mean±s.e.m. (_n_=6 mice per group); *P<0.05; **P<0.01. BTZ, Bortezomib. (B) Frozen liver tissue sections stained with Oil-red O for hepatic lipid contents after the Bortezomib treatment (magnification × 400). (C) Levels of intracellular liver triglycerides after the Bortezomib treatment. (D) Western blot analysis of C/EBPβ, C/EBPδ, PPARγ, and CHOP in the liver. Levels of β-actin protein were included as internal controls. The values below the gels represent normalized protein signal intensities. (E–I) Plasmid DNA vector (100 μg/mouse) expressing an hFVIII transgene encoding a misfolding-prone hFVIII (hFVIII) was transferred into the _Ire1_α-null (Ire1_α_Hepfe/−) and control (Ire1_α_Hepfe/+) mice at 3 months of age through hydrodynamic tail-vein injection. Liver tissues and blood plasma samples were collected at 24 h after the injection. (E) Levels of the hFVIII antigen in liver cellular lysates or plasma of _Ire1_α-null and control mice after the injection of the hFVIII expression vector. Each bar denotes the mean level of hFVIII antigen in plasma (ng/ml) or in liver tissue (ng/mg liver protein)±s.e.m. (_n_=3 mice per group). *P<0.05. (F) Quantitative real-time RT–PCR analysis for expression of hFVIII mRNA in the liver of _Ire1_α-null and control mice after the injection of the hFVIII expression vector. Expression values were normalized to β-actin mRNA. Fold changes of mRNA levels were determined by comparison to the expression level in the uninjected control. Each bar denotes the mean±s.e.m. (_n_=3 mice per group); **P<0.01. UI, uninjected control. (G) Frozen liver tissue sections stained with Oil-red O for hepatic lipid contents after the injection of the expression vector for hFVIII (magnification × 400). (H) Levels of hepatic and plasma triglycerides in the mice after the injection of hFVIII expression vector. (I) Quantitative real-time RT–PCR analysis for expression of lipogenic genes in the liver of _Ire1_α-null and control mice after the injection of the hFVIII expression vector. Expression values were normalized to β-actin mRNA. Fold changes of mRNA levels were determined by comparison to the expression level in one of the controls. Each bar denotes the mean±s.e.m. (_n_=3 mice per group); **P<0.01.
Similar articles
- IRE1α prevents hepatic steatosis by processing and promoting the degradation of select microRNAs.
Wang JM, Qiu Y, Yang Z, Kim H, Qian Q, Sun Q, Zhang C, Yin L, Fang D, Back SH, Kaufman RJ, Yang L, Zhang K. Wang JM, et al. Sci Signal. 2018 May 15;11(530):eaao4617. doi: 10.1126/scisignal.aao4617. Sci Signal. 2018. PMID: 29764990 Free PMC article. - Fibroblast growth factor 21 is regulated by the IRE1α-XBP1 branch of the unfolded protein response and counteracts endoplasmic reticulum stress-induced hepatic steatosis.
Jiang S, Yan C, Fang QC, Shao ML, Zhang YL, Liu Y, Deng YP, Shan B, Liu JQ, Li HT, Yang L, Zhou J, Dai Z, Liu Y, Jia WP. Jiang S, et al. J Biol Chem. 2014 Oct 24;289(43):29751-65. doi: 10.1074/jbc.M114.565960. Epub 2014 Aug 28. J Biol Chem. 2014. PMID: 25170079 Free PMC article. - Phosphorylation at Ser724 of the ER stress sensor IRE1α governs its activation state and limits ER stress-induced hepatosteatosis.
Li Y, Huang S, Wang J, Dai J, Cai J, Yan S, Huang Z, He S, Wang P, Liu J, Liu Y. Li Y, et al. J Biol Chem. 2022 Jun;298(6):101997. doi: 10.1016/j.jbc.2022.101997. Epub 2022 Apr 29. J Biol Chem. 2022. PMID: 35500653 Free PMC article. - Quality Control in the Endoplasmic Reticulum: Crosstalk between ERAD and UPR pathways.
Hwang J, Qi L. Hwang J, et al. Trends Biochem Sci. 2018 Aug;43(8):593-605. doi: 10.1016/j.tibs.2018.06.005. Epub 2018 Jun 29. Trends Biochem Sci. 2018. PMID: 30056836 Free PMC article. Review. - Emerging roles for the ER stress sensor IRE1α in metabolic regulation and disease.
Huang S, Xing Y, Liu Y. Huang S, et al. J Biol Chem. 2019 Dec 6;294(49):18726-18741. doi: 10.1074/jbc.REV119.007036. Epub 2019 Oct 30. J Biol Chem. 2019. PMID: 31666338 Free PMC article. Review.
Cited by
- IRE1 prevents endoplasmic reticulum membrane permeabilization and cell death under pathological conditions.
Kanekura K, Ma X, Murphy JT, Zhu LJ, Diwan A, Urano F. Kanekura K, et al. Sci Signal. 2015 Jun 23;8(382):ra62. doi: 10.1126/scisignal.aaa0341. Sci Signal. 2015. PMID: 26106220 Free PMC article. - IRE1A Stimulates Hepatocyte-Derived Extracellular Vesicles That Promote Inflammation in Mice With Steatohepatitis.
Dasgupta D, Nakao Y, Mauer AS, Thompson JM, Sehrawat TS, Liao CY, Krishnan A, Lucien F, Guo Q, Liu M, Xue F, Fukushima M, Katsumi T, Bansal A, Pandey MK, Maiers JL, DeGrado T, Ibrahim SH, Revzin A, Pavelko KD, Barry MA, Kaufman RJ, Malhi H. Dasgupta D, et al. Gastroenterology. 2020 Oct;159(4):1487-1503.e17. doi: 10.1053/j.gastro.2020.06.031. Epub 2020 Jun 20. Gastroenterology. 2020. PMID: 32574624 Free PMC article. - Endoplasmic Reticulum Stress and Autophagy in the Pathogenesis of Non-alcoholic Fatty Liver Disease (NAFLD): Current Evidence and Perspectives.
Flessa CM, Kyrou I, Nasiri-Ansari N, Kaltsas G, Papavassiliou AG, Kassi E, Randeva HS. Flessa CM, et al. Curr Obes Rep. 2021 Jun;10(2):134-161. doi: 10.1007/s13679-021-00431-3. Epub 2021 Mar 22. Curr Obes Rep. 2021. PMID: 33751456 Review. - Protein Quality Control and Lipid Droplet Metabolism.
Roberts MA, Olzmann JA. Roberts MA, et al. Annu Rev Cell Dev Biol. 2020 Oct 6;36:115-139. doi: 10.1146/annurev-cellbio-031320-101827. Annu Rev Cell Dev Biol. 2020. PMID: 33021827 Free PMC article. Review. - ER-associated degradation in health and disease - from substrate to organism.
Bhattacharya A, Qi L. Bhattacharya A, et al. J Cell Sci. 2019 Dec 2;132(23):jcs232850. doi: 10.1242/jcs.232850. J Cell Sci. 2019. PMID: 31792042 Free PMC article. Review.
References
- Allain CC, Poon LS, Chan CS, Richmond W, Fu PC (1974) Enzymatic determination of total serum cholesterol. Clin Chem 20: 470–475 - PubMed
- Berry MN, Edwards AM, Barritt GJ (1991) Isolated hepatocytes preparation, properties and applications. In Laboratory Techniques in Biochemistry and Molecular Biology, pp 59–81. New York: Elsevier Science Publishing Co., Inc
- Brasaemle DL, Barber T, Wolins NE, Serrero G, Blanchette-Mackie EJ, Londos C (1997) Adipose differentiation-related protein is an ubiquitously expressed lipid storage droplet-associated protein. J Lipid Res 38: 2249–2263 - PubMed
- Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D (2002) IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 415: 92–96 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK088227/DK/NIDDK NIH HHS/United States
- P01 HL057346/HL/NHLBI NIH HHS/United States
- R01 GM062896/GM/NIGMS NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R37 DK042394/DK/NIDDK NIH HHS/United States
- R01 DK088227-01/DK/NIDDK NIH HHS/United States
- 5P60DK20572/DK/NIDDK NIH HHS/United States
- HL057346/HL/NHLBI NIH HHS/United States
- 1R21ES017829-01A1/ES/NIEHS NIH HHS/United States
- DK042394/DK/NIDDK NIH HHS/United States
- HL052173/HL/NHLBI NIH HHS/United States
- R03 MH089782-02/MH/NIMH NIH HHS/United States
- P60 DK020572/DK/NIDDK NIH HHS/United States
- R21 ES017829/ES/NIEHS NIH HHS/United States
- R01 DK042394/DK/NIDDK NIH HHS/United States
- R01 HL052173/HL/NHLBI NIH HHS/United States
- R01 HL052173-14A1/HL/NHLBI NIH HHS/United States
- R03 MH089782/MH/NIMH NIH HHS/United States
- R37 DK042394-14/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases