Comparative cardiovascular effects of thiazolidinediones: systematic review and meta-analysis of observational studies - PubMed (original) (raw)
Review
Comparative cardiovascular effects of thiazolidinediones: systematic review and meta-analysis of observational studies
Yoon Kong Loke et al. BMJ. 2011.
Abstract
Objective: To determine the comparative effects of the thiazolidinediones (rosiglitazone and pioglitazone) on myocardial infarction, congestive heart failure, and mortality in patients with type 2 diabetes.
Design: Systematic review and meta-analysis of observational studies.
Data sources: Searches of Medline and Embase in September 2010.
Study selection: Observational studies that directly compared the risk of cardiovascular outcomes for rosiglitazone and pioglitazone among patients with type 2 diabetes mellitus were included.
Data extraction: Random effects meta-analysis (inverse variance method) was used to calculate the odds ratios for cardiovascular outcomes with thiazolidinedione use. The I(2 )statistic was used to assess statistical heterogeneity.
Results: Cardiovascular outcomes from 16 observational studies (4 case-control studies and 12 retrospective cohort studies), including 810,000 thiazolidinedione users, were evaluated after a detailed review of 189 citations. Compared with pioglitazone, use of rosiglitazone was associated with a statistically significant increase in the odds of myocardial infarction (n = 15 studies; odds ratio 1.16, 95% confidence interval 1.07 to 1.24; P < 0.001; I(2) = 46%), congestive heart failure (n = 8; 1.22, 1.14 to 1.31; P < 0.001; I(2) = 37%), and death (n = 8; 1.14, 1.09 to 1.20; P < 0.001; I(2) = 0%). Numbers needed to treat to harm (NNH), depending on the population at risk, suggest 170 excess myocardial infarctions, 649 excess cases of heart failure, and 431 excess deaths for every 100,000 patients who receive rosiglitazone rather than pioglitazone.
Conclusion: Among patients with type 2 diabetes, use of rosiglitazone is associated with significantly higher odds of congestive heart failure, myocardial infarction, and death relative to pioglitazone in real world settings.
Conflict of interest statement
Competing interests: All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi\_disclosure.pdf (available on request from the corresponding author) and declare that (1) they have no relationships with any company that might have an interest in the submitted work in the previous three years; (2) their spouses, partners, or children have no financial relationships that may be relevant to the submitted work; and (4) they have no non-financial interests that may be relevant to the submitted work.
Figures
Fig 1 Flow diagram of process of selection of articles for meta-analysis. GSK=GlaxoSmithKline
Fig 2 Meta-analysis of odds ratio for myocardial infarction with rosiglitazone versus pioglitazone
Fig 3 Meta-analysis of odds ratio for heart failure with rosiglitazone versus pioglitazone
Fig 4 Meta-analysis of odds ratio for overall mortality with rosiglitazone versus pioglitazone
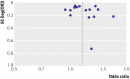
Fig 5 Funnel plot based on odds ratio for myocardial infarction and study precision
Comment in
- What have we learnt from the rosiglitazone saga?
Montori VM, Shah ND. Montori VM, et al. BMJ. 2011 Mar 17;342:d1354. doi: 10.1136/bmj.d1354. BMJ. 2011. PMID: 21415102 No abstract available. - Rosiglitazone saga. Demonisation of rosiglitazone.
Heazlewood VJ. Heazlewood VJ. BMJ. 2011 Apr 21;342:d2571; author reply d2573. doi: 10.1136/bmj.d2571. BMJ. 2011. PMID: 21511791 No abstract available. - ACP Journal Club. Review: rosiglitazone increases risk for cardiovascular outcomes and mortality compared with pioglitazone in type 2 diabetes.
Deedwania P. Deedwania P. Ann Intern Med. 2011 Aug 16;155(4):JC2-12. doi: 10.7326/0003-4819-155-4-201108160-02012. Ann Intern Med. 2011. PMID: 21844545 No abstract available.
Similar articles
- Risk of acute myocardial infarction, stroke, heart failure, and death in elderly Medicare patients treated with rosiglitazone or pioglitazone.
Graham DJ, Ouellet-Hellstrom R, MaCurdy TE, Ali F, Sholley C, Worrall C, Kelman JA. Graham DJ, et al. JAMA. 2010 Jul 28;304(4):411-8. doi: 10.1001/jama.2010.920. Epub 2010 Jun 28. JAMA. 2010. PMID: 20584880 - Risk of cardiovascular disease and all cause mortality among patients with type 2 diabetes prescribed oral antidiabetes drugs: retrospective cohort study using UK general practice research database.
Tzoulaki I, Molokhia M, Curcin V, Little MP, Millett CJ, Ng A, Hughes RI, Khunti K, Wilkins MR, Majeed A, Elliott P. Tzoulaki I, et al. BMJ. 2009 Dec 3;339:b4731. doi: 10.1136/bmj.b4731. BMJ. 2009. PMID: 19959591 Free PMC article. - Adverse cardiovascular events during treatment with pioglitazone and rosiglitazone: population based cohort study.
Juurlink DN, Gomes T, Lipscombe LL, Austin PC, Hux JE, Mamdani MM. Juurlink DN, et al. BMJ. 2009 Aug 18;339:b2942. doi: 10.1136/bmj.b2942. BMJ. 2009. PMID: 19690342 Free PMC article. - Rosiglitazone and cardiovascular risk.
Kaul S, Diamond GA. Kaul S, et al. Curr Atheroscler Rep. 2008 Oct;10(5):398-404. doi: 10.1007/s11883-008-0062-7. Curr Atheroscler Rep. 2008. PMID: 18706281 Review. - Thiazolidinediones in type 2 diabetes: a cardiology perspective.
Khanderia U, Pop-Busui R, Eagle KA. Khanderia U, et al. Ann Pharmacother. 2008 Oct;42(10):1466-74. doi: 10.1345/aph.1K666. Epub 2008 Sep 2. Ann Pharmacother. 2008. PMID: 18698014 Review.
Cited by
- A drug mix and dose decision algorithm for individualized type 2 diabetes management.
Nambiar M, Bee YM, Chan YE, Ho Mien I, Guretno F, Carmody D, Lee PC, Chia SY, Salim NNM, Krishnaswamy P. Nambiar M, et al. NPJ Digit Med. 2024 Sep 17;7(1):254. doi: 10.1038/s41746-024-01230-5. NPJ Digit Med. 2024. PMID: 39289474 Free PMC article. - Dysregulated cellular metabolism in atherosclerosis: mediators and therapeutic opportunities.
Stroope C, Nettersheim FS, Coon B, Finney AC, Schwartz MA, Ley K, Rom O, Yurdagul A Jr. Stroope C, et al. Nat Metab. 2024 Apr;6(4):617-638. doi: 10.1038/s42255-024-01015-w. Epub 2024 Mar 26. Nat Metab. 2024. PMID: 38532071 Free PMC article. Review. - Beyond Blood Sugar: Investigating the Cardiovascular Effects of Antidiabetic Drugs.
Ahmad BA, Sanghani IM, Sayabugari R, Biju H, Siddegowda A, Ittiachen Kinattingal M, Yartha SGR, Gaonkar PM, Andrabi SS, Vaghamashi YK, Korwar A. Ahmad BA, et al. Cureus. 2023 Oct 2;15(10):e46373. doi: 10.7759/cureus.46373. eCollection 2023 Oct. Cureus. 2023. PMID: 37920618 Free PMC article. Review. - Utility of Hypoglycemic Agents to Treat Asthma with Comorbid Obesity.
Ge D, Foer D, Cahill KN. Ge D, et al. Pulm Ther. 2023 Mar;9(1):71-89. doi: 10.1007/s41030-022-00211-x. Epub 2022 Dec 27. Pulm Ther. 2023. PMID: 36575356 Free PMC article. Review. - Cardiovascular and renal outcomes with sodium glucose co-transporter 2 inhibitors in patients with type 2 diabetes mellitus: A system review and network meta-analysis.
Tian L, Ai S, Zheng H, Yang H, Zhou M, Tang J, Liu W, Zhao W, Wang Y. Tian L, et al. Front Pharmacol. 2022 Nov 24;13:986186. doi: 10.3389/fphar.2022.986186. eCollection 2022. Front Pharmacol. 2022. PMID: 36506550 Free PMC article.
References
- Gale EA. Lessons from the glitazones: a story of drug development. Lancet 2001;357:1870-5. - PubMed
- Nissen SE, Wolski K, Topol EJ. Effect of muraglitazar on death and major adverse cardiovascular events in patients with type 2 diabetes mellitus. JAMA 2005;294:2581-6. - PubMed
- Nissen SE, Wolski K. Rosiglitazone revisited: an updated meta-analysis of risk for myocardial infarction and cardiovascular mortality. Arch Intern Med 2010;170:1191-201. - PubMed
- Singh S, Loke YK, Furberg CD. Long-term risk of cardiovascular events with rosiglitazone: a meta-analysis. JAMA 2007;298:1189-95. - PubMed
- Lincoff AM, Wolski K, Nicholls SJ, Nissen SE. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. JAMA 2007;298:1180-8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical