Co-occupancy by multiple cardiac transcription factors identifies transcriptional enhancers active in heart - PubMed (original) (raw)
Co-occupancy by multiple cardiac transcription factors identifies transcriptional enhancers active in heart
Aibin He et al. Proc Natl Acad Sci U S A. 2011.
Abstract
Identification of genomic regions that control tissue-specific gene expression is currently problematic. ChIP and high-throughput sequencing (ChIP-seq) of enhancer-associated proteins such as p300 identifies some but not all enhancers active in a tissue. Here we show that co-occupancy of a chromatin region by multiple transcription factors (TFs) identifies a distinct set of enhancers. GATA-binding protein 4 (GATA4), NK2 transcription factor-related, locus 5 (NKX2-5), T-box 5 (TBX5), serum response factor (SRF), and myocyte-enhancer factor 2A (MEF2A), here referred to as "cardiac TFs," have been hypothesized to collaborate to direct cardiac gene expression. Using a modified ChIP-seq procedure, we defined chromatin occupancy by these TFs and p300 genome wide and provided unbiased support for this hypothesis. We used this principle to show that co-occupancy of a chromatin region by multiple TFs can be used to identify cardiac enhancers. Of 13 such regions tested in transient transgenic embryos, seven (54%) drove cardiac gene expression. Among these regions were three cardiac-specific enhancers of Gata4, Srf, and swItch/sucrose nonfermentable-related, matrix-associated, actin-dependent regulator of chromatin, subfamily d, member 3 (Smarcd3), an epigenetic regulator of cardiac gene expression. Multiple cardiac TFs and p300-bound regions were associated with cardiac-enriched genes and with functional annotations related to heart development. Importantly, the large majority (1,375/1,715) of loci bound by multiple cardiac TFs did not overlap loci bound by p300. Our data identify thousands of prospective cardiac regulatory sequences and indicate that multiple TF co-occupancy of a genomic region identifies developmentally relevant enhancers that are largely distinct from p300-associated enhancers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
In vivo TF binding motifs. De novo motif discovery of in vivo motifs by MEME and Weeder using the top 500 peaks of ChIP-seq data. All MEME E-values were less than 10−60. For TBX5, high GC-content peaks were excluded. Motifs found by de novo discovery were compared with available consensus and optimal in vitro motifs from JASPAR, UniPROBE, or the indicated reference. Dashed boxes highlight differences between in vivo and in vitro motifs.
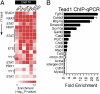
Fig. 2.
Expansion of the cardiac TF interaction network by motif enrichment analysis. (A) Heat map showing statistical enrichment of selected JASPAR and TRANSFAC motifs among top 500 peaks bound by cardiac TF. A heat map of all analyzed motifs is shown in
SI Appendix, Fig. S5
. (B) TEAD1 ChIP-qPCR assay of cardiac TF peaks containing predicted TEAD1 motifs. Fold enrichment indicates TEAD1 compared with IgG1 ChIP and normalized to Actin-β (Actb) intronic control. Filled bars indicate greater than twofold enrichment (dotted line). Actn4, actinin α4; Afap1, actin filament-associated protein 1; Cap2, adenylate cyclase-associated protein, 2; Cdh1, cadherin 1 type 1; Cdh2, cadherin 2; Chd2, chromodomain helicase DNA binding protein 2; Col4a3, collagen type IV, α3; Fgf12, fibroblast growth factor 12; Galnt2, UDP-_N_-acetyl-α-
d
-galactosamine:polypeptide _N_-acetylgalactosaminyltransferase 2; Myst4, MYST histone acetyltransferase (monocytic leukemia) 4; Rbpms, RNA binding protein gene with multiple splicing; Scn10a, sodium channel, voltage-gated type X α subunit; Tcf3, transcription factor 3.
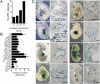
Fig. 3.
Genomic regions co-occupied by multiple cardiac TFs direct cardiac gene expression. (A) Genes with a higher cardiac enrichment score (cardiac expression/average expression in other tissues) were associated more frequently with MTLs. (B) Enhancer activity in vitro. Enhancers cloned upstream of hsp68-lacZ were transfected into neonatal rat cardiomyocytes or cardiac fibroblasts along with pGL3-luc. Ratio of LacZ activity in neonatal rat ventricular myocytes (NRVM) to fibroblasts was plotted after normalization to luciferase activity. n = 3. *P < 0.05; **P < 0.01; ***P < 0.001. NS, not significant. (C) Enhancer–hsp68–lacZ constructs were used to generate E10.5 transgenic embryos. Representative Xgal-stained embryos are shown. Numbers indicate embryos with cardiac expression over the total PCR+ embryos. Arrowheads indicate myocardial expression. Black arrows indicate activity in endocardium and endocardial cushions. PE, proepicardium; SHF, second heart field. (White scale bars: 400 μm; black scale bars: 200 μm.)
Fig. 4.
GATA4 and TBX5 binding sites are required for Smarcd3 _−_1497 activity. (A) Validation of GATA4 occupancy of _Smarcd3 −_1497 by ChIP-qPCR from mouse heart at the indicated developmental stages. E, embryonic; P, postnatal. (B) Activity of _Smarcd3 −_1497 enhancers containing mutation of GATA4 (G4m), TBX5 (T5m), or both motifs indicated in
SI Appendix, Fig. S6_D_
. Arrow indicates residual activity in outflow tract. Yellow arrowhead indicates loss of activity in cardiac chambers. Numbers indicate Xgal+ and PCR+ embryos. (Scale bars: 500 μm.)
Fig. 5.
MultiTF and p300 binding mark distinct sets of enhancers. (A) p300 frequently co-occupied genomic loci with cardiac TF, most notably GATA4. (B) Genes with higher cardiac enrichment scores were associated more frequently with p300. (C) The preponderance of multiTF and p300 enhancers did not overlap. (D) MultiTF and p300 genes were more highly expressed in HL1 cells than were genes that lacked these enhancers (P < 10−16), but expression levels of multiTF and p300 genes were indistinguishable. Gene expression is indicated in log2 scale. (E) MultiTF enhancers were located more proximal to the TSS than p300 enhancers. (F) Gene Ontology (GO) term analysis of MultiTF+/p300− and MultiTF−/p300+ enhancers. Top 10 terms, fraction of positive genes within the set, and Benjamini–Hochberg false discovery rate (FDR) are shown for each class of enhancers.
Similar articles
- A reference map of murine cardiac transcription factor chromatin occupancy identifies dynamic and conserved enhancers.
Akerberg BN, Gu F, VanDusen NJ, Zhang X, Dong R, Li K, Zhang B, Zhou B, Sethi I, Ma Q, Wasson L, Wen T, Liu J, Dong K, Conlon FL, Zhou J, Yuan GC, Zhou P, Pu WT. Akerberg BN, et al. Nat Commun. 2019 Oct 28;10(1):4907. doi: 10.1038/s41467-019-12812-3. Nat Commun. 2019. PMID: 31659164 Free PMC article. - In Vivo Dissection of Chamber-Selective Enhancers Reveals Estrogen-Related Receptor as a Regulator of Ventricular Cardiomyocyte Identity.
Cao Y, Zhang X, Akerberg BN, Yuan H, Sakamoto T, Xiao F, VanDusen NJ, Zhou P, Sweat ME, Wang Y, Prondzynski M, Chen J, Zhang Y, Wang P, Kelly DP, Pu WT. Cao Y, et al. Circulation. 2023 Mar 14;147(11):881-896. doi: 10.1161/CIRCULATIONAHA.122.061955. Epub 2023 Jan 27. Circulation. 2023. PMID: 36705030 Free PMC article. - Enhancer identification in mouse embryonic stem cells using integrative modeling of chromatin and genomic features.
Chen CY, Morris Q, Mitchell JA. Chen CY, et al. BMC Genomics. 2012 Apr 26;13:152. doi: 10.1186/1471-2164-13-152. BMC Genomics. 2012. PMID: 22537144 Free PMC article. - Epigenetic mechanisms in cardiac development and disease.
Vallaster M, Vallaster CD, Wu SM. Vallaster M, et al. Acta Biochim Biophys Sin (Shanghai). 2012 Jan;44(1):92-102. doi: 10.1093/abbs/gmr090. Acta Biochim Biophys Sin (Shanghai). 2012. PMID: 22194017 Free PMC article. Review. - Cardiac Transcription Factors and Regulatory Networks.
Grunert M, Dorn C, Rickert-Sperling S. Grunert M, et al. Adv Exp Med Biol. 2024;1441:295-311. doi: 10.1007/978-3-031-44087-8_16. Adv Exp Med Biol. 2024. PMID: 38884718 Review.
Cited by
- The epigenome and its role in diabetes.
Waki H, Yamauchi T, Kadowaki T. Waki H, et al. Curr Diab Rep. 2012 Dec;12(6):673-85. doi: 10.1007/s11892-012-0328-x. Curr Diab Rep. 2012. PMID: 23070559 Review. - Tbx2 and Tbx3 induce atrioventricular myocardial development and endocardial cushion formation.
Singh R, Hoogaars WM, Barnett P, Grieskamp T, Rana MS, Buermans H, Farin HF, Petry M, Heallen T, Martin JF, Moorman AF, 't Hoen PA, Kispert A, Christoffels VM. Singh R, et al. Cell Mol Life Sci. 2012 Apr;69(8):1377-89. doi: 10.1007/s00018-011-0884-2. Epub 2011 Dec 1. Cell Mol Life Sci. 2012. PMID: 22130515 Free PMC article. - Severe myopathy in mice lacking the MEF2/SRF-dependent gene leiomodin-3.
Cenik BK, Garg A, McAnally JR, Shelton JM, Richardson JA, Bassel-Duby R, Olson EN, Liu N. Cenik BK, et al. J Clin Invest. 2015 Apr;125(4):1569-78. doi: 10.1172/JCI80115. Epub 2015 Mar 16. J Clin Invest. 2015. PMID: 25774500 Free PMC article. - HAND transcription factors cooperatively specify the aorta and pulmonary trunk.
Vincentz JW, Firulli BA, Toolan KP, Osterwalder M, Pennacchio LA, Firulli AB. Vincentz JW, et al. Dev Biol. 2021 Aug;476:1-10. doi: 10.1016/j.ydbio.2021.03.011. Epub 2021 Mar 20. Dev Biol. 2021. PMID: 33757801 Free PMC article. - MEF2B mutations in non-Hodgkin lymphoma dysregulate cell migration by decreasing MEF2B target gene activation.
Pon JR, Wong J, Saberi S, Alder O, Moksa M, Grace Cheng SW, Morin GB, Hoodless PA, Hirst M, Marra MA. Pon JR, et al. Nat Commun. 2015 Aug 6;6:7953. doi: 10.1038/ncomms8953. Nat Commun. 2015. PMID: 26245647 Free PMC article.
References
- Heintzman ND, et al. Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat Genet. 2007;39:311–318. - PubMed
- Naya FJ, et al. Mitochondrial deficiency and cardiac sudden death in mice lacking the MEF2A transcription factor. Nat Med. 2002;8:1303–1309. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UM1 HL098166/HL/NHLBI NIH HHS/United States
- HL095712/HL/NHLBI NIH HHS/United States
- R01 HL095712/HL/NHLBI NIH HHS/United States
- U01 HL098166/HL/NHLBI NIH HHS/United States
- HL098166/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous