The adaptor protein TRADD is essential for TNF-like ligand 1A/death receptor 3 signaling - PubMed (original) (raw)
The adaptor protein TRADD is essential for TNF-like ligand 1A/death receptor 3 signaling
Yelena L Pobezinskaya et al. J Immunol. 2011.
Abstract
TNFR-associated death domain protein (TRADD) is a key effector protein of TNFR1 signaling. However, the role of TRADD in other death receptor (DR) signaling pathways, including DR3, has not been completely characterized. Previous studies using overexpression systems suggested that TRADD is recruited to the DR3 complex in response to the DR3 ligand, TNF-like ligand 1A (TL1A), indicating a possible role in DR3 signaling. Using T cells from TRADD knockout mice, we demonstrate in this study that the response of both CD4(+) and CD8(+) T cells to TL1A is dependent upon the presence of TRADD. TRADD knockout T cells therefore lack the appropriate proliferative response to TL1A. Moreover, in the absence of TRADD, both the stimulation of MAPK signaling and activation of NF-κB in response to TL1A are dramatically reduced. Unsurprisingly, TRADD is required for recruitment of receptor interacting protein 1 and TNFR-associated factor 2 to the DR3 signaling complex and for the ubiquitination of receptor interacting protein 1. Thus, our findings definitively establish an essential role of TRADD in DR3 signaling.
Figures
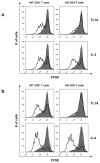
Figure 1. TRADD-deficient T cells do not proliferate in response to TL1A treatment
CFSE labeled wild type and knockout T cells were treated with TL1A (50 μg/ml; top panels) or IL-2 (100U/ml; bottom panels) for 72 hours, then stained with anti-CD4 or anti-CD8 antibodies and analyzed by flow cytometry. Histograms are gated on CD4+ (a) and CD8+ cells (b).
Figure 2. TRADD is required for MAP kinase and NFκB activation
a–c, Western blot analysis of lysates of wild type and TRADD knockout CD4+ (a), CD8+ (b) T cells and pan-T cells (c) treated with TL1A (50 μg/ml) (a,b) for the indicated times or PMA (100nM) (c). d, Nuclear extracts of wild type and knockout T cells treated with TNFα (30 ng/ml) and TL1A (50 μg/ml) for indicated times were analyzed by EMSA.
Figure 3. TRADD is required for formation of the functional DR3 signaling complex
Cell extracts from wild type and TRADD knockout T cells treated with TL1A (50 μg/ml) for 5 min were immunoprecipitated with anti-DR3 (ip:DR3) and analyzed by western blotting. Input, 2% of extract before immunoprecipitation. Ub-, ubiquitinated.
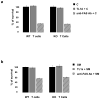
Figure 4. TL1A does not induce apoptosis in primary T cells
MTS assay of the viability of wild type and TRADD knockout T cells treated for 24 hours with cycloheximide (C) alone (10μg/ml), cycloheximide and TL1A (50 μg/ml) or cycloheximide and anti-FAS (10ng/ml) (a) and SMAC mimetic (SM) alone (10nM), SMAC mimetic and TL1A (50 μg/ml) or SMAC mimetic and anti-FAS (10ng/ml) (b). Error bars shown represent SEM.
Similar articles
- TL1A and DR3, a TNF family ligand-receptor pair that promotes lymphocyte costimulation, mucosal hyperplasia, and autoimmune inflammation.
Meylan F, Richard AC, Siegel RM. Meylan F, et al. Immunol Rev. 2011 Nov;244(1):188-96. doi: 10.1111/j.1600-065X.2011.01068.x. Immunol Rev. 2011. PMID: 22017439 Free PMC article. Review. - Tumor Necrosis Factor-like Cytokine TL1A and Its Receptors DR3 and DcR3: Important New Factors in Mucosal Homeostasis and Inflammation.
Siakavellas SI, Bamias G. Siakavellas SI, et al. Inflamm Bowel Dis. 2015 Oct;21(10):2441-52. doi: 10.1097/MIB.0000000000000492. Inflamm Bowel Dis. 2015. PMID: 26099067 Review. - The TNF-family receptor DR3 is essential for diverse T cell-mediated inflammatory diseases.
Meylan F, Davidson TS, Kahle E, Kinder M, Acharya K, Jankovic D, Bundoc V, Hodges M, Shevach EM, Keane-Myers A, Wang EC, Siegel RM. Meylan F, et al. Immunity. 2008 Jul 18;29(1):79-89. doi: 10.1016/j.immuni.2008.04.021. Epub 2008 Jun 19. Immunity. 2008. PMID: 18571443 Free PMC article. - Activation of the DR3-TL1A Axis in Donor Mice Leads to Regulatory T Cell Expansion and Activation With Reduction in Graft-Versus-Host Disease.
Mavers M, Simonetta F, Nishikii H, Ribado JV, Maas-Bauer K, Alvarez M, Hirai T, Turkoz M, Baker J, Negrin RS. Mavers M, et al. Front Immunol. 2019 Jul 17;10:1624. doi: 10.3389/fimmu.2019.01624. eCollection 2019. Front Immunol. 2019. PMID: 31379829 Free PMC article. - Beyond tumor necrosis factor receptor: TRADD signaling in toll-like receptors.
Chen NJ, Chio II, Lin WJ, Duncan G, Chau H, Katz D, Huang HL, Pike KA, Hao Z, Su YW, Yamamoto K, de Pooter RF, Zúñiga-Pflücker JC, Wakeham A, Yeh WC, Mak TW. Chen NJ, et al. Proc Natl Acad Sci U S A. 2008 Aug 26;105(34):12429-34. doi: 10.1073/pnas.0806585105. Epub 2008 Aug 21. Proc Natl Acad Sci U S A. 2008. PMID: 18719121 Free PMC article.
Cited by
- The ever-expanding role of cytokine receptor DR3 in T cells.
Liman N, Lanasa D, Meylan F, Park JH. Liman N, et al. Cytokine. 2024 Apr;176:156540. doi: 10.1016/j.cyto.2024.156540. Epub 2024 Feb 15. Cytokine. 2024. PMID: 38359559 Review. - TL1A induces apoptosis via DR3 in grass carp (Ctenopharyngodon idella).
Chen K, Xiu Q, Min Q, Cheng X, Xiao H, Jia Z, Feng J, Shi Y, Zhuo Q, Wang J, Zou J. Chen K, et al. Fish Shellfish Immunol Rep. 2023 Mar 15;4:100090. doi: 10.1016/j.fsirep.2023.100090. eCollection 2023 Dec. Fish Shellfish Immunol Rep. 2023. PMID: 36970231 Free PMC article. - Functional roles in cell signaling of adaptor protein TRADD from a structural perspective.
Li Z, Yuan W, Lin Z. Li Z, et al. Comput Struct Biotechnol J. 2020 Oct 16;18:2867-2876. doi: 10.1016/j.csbj.2020.10.008. eCollection 2020. Comput Struct Biotechnol J. 2020. PMID: 33163147 Free PMC article. Review. - TNFSF15 Promotes Antimicrobial Pathways in Human Macrophages and These Are Modulated by TNFSF15 Disease-Risk Variants.
Sun R, Hedl M, Abraham C. Sun R, et al. Cell Mol Gastroenterol Hepatol. 2021;11(1):249-272. doi: 10.1016/j.jcmgh.2020.08.003. Epub 2020 Aug 19. Cell Mol Gastroenterol Hepatol. 2021. PMID: 32827707 Free PMC article. - Arg-GlcNAcylation on TRADD by NleB and SseK1 Is Crucial for Bacterial Pathogenesis.
Xue J, Hu S, Huang Y, Zhang Q, Yi X, Pan X, Li S. Xue J, et al. Front Cell Dev Biol. 2020 Jul 17;8:641. doi: 10.3389/fcell.2020.00641. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32766249 Free PMC article.
References
- Lavrik I, Golks A, Krammer PH. Death receptor signaling. J Cell Sci. 2005;118:265–7. - PubMed
- Kitson J, Raven T, Jiang YP, et al. A death-domain-containing receptor that mediates apoptosis. Nature. 1996;384:372–5. - PubMed
- Bodmer JL, Burns K, Schneider P, et al. TRAMP, a novel apoptosis-mediating receptor with sequence homology to tumor necrosis factor receptor 1 and Fas(Apo-1/CD95) Immunity. 1997;6:79–88. - PubMed
- Chinnaiyan AM, O'Rourke K, Yu GL, et al. Signal transduction by DR3, a death domain-containing receptor related to TNFR-1 and CD95. Science. 1996;274:990–2. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials