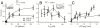Dendritic cell chemotaxis in 3D under defined chemokine gradients reveals differential response to ligands CCL21 and CCL19 - PubMed (original) (raw)
Comparative Study
. 2011 Apr 5;108(14):5614-9.
doi: 10.1073/pnas.1014920108. Epub 2011 Mar 21.
Affiliations
- PMID: 21422278
- PMCID: PMC3078419
- DOI: 10.1073/pnas.1014920108
Comparative Study
Dendritic cell chemotaxis in 3D under defined chemokine gradients reveals differential response to ligands CCL21 and CCL19
Ulrike Haessler et al. Proc Natl Acad Sci U S A. 2011.
Abstract
Dendritic cell (DC) homing to the lymphatics and positioning within the lymph node is important for adaptive immunity, and is regulated by gradients of CCL19 and CCL21, ligands for CCR7. Despite the importance of DC chemotaxis, it is not well understood how DCs interpret gradients of these chemokines in a complex 3D microenvironment. Here, we use a microfluidic device that allows rapid establishment of stable gradients in 3D matrices to show that DC chemotaxis in 3D can respond to CCR7 ligand gradients as small as 0.4%, which helps explain how DCs sense lymphatic vessels in an environment where broadcast distance for chemokine diffusion is hindered by convective flows into the vessel. Interestingly, DCs displayed similar sensitivities to both chemokines at small gradients (≤ 60 nM/mm), but migrated more efficiently towards higher gradients of CCL21, which unlike CCL19 binds strongly to matrix proteoglycans and signals without the need for internalization. Furthermore, cells preferentially migrated towards CCL21 when exposed to equal and opposite gradients of CCL21 and CCL19 simultaneously, even when matrix-binding of CCL21 was prevented. Although these ligands have similar binding affinity to CCR7, our results demonstrate that, in a 3D environment, CCL21 is a more potent directional cue for DC migration than CCL19. These findings provide new quantitative insight into DC chemotaxis in a physiological 3D environment and suggest how CCL19 and CCL21 may signal differently to fine-tune DC homing and positioning within the lymphatic system. These results also have broad relevance to other systems of cell chemotaxis, which remain poorly understood in the 3D context.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
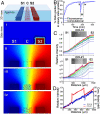
Fig. 1.
Establishment and characterization of matrix bound and soluble protein gradients in a microfluidic device. (A) Schematic view of one functional unit of the microfluidic device. The cell-matrix mixture was seeded in the center channel (C), while the chemokines/buffers flowed along the side channels (S1 and S2) to create chemical gradients across the center channel (i–iv). Computational results of CCL19 concentration gradients at various times, red and blue correspond to maximum and minimum concentrations, respectively. (i) At t = 0, chemokine solutions were pumped through the side channels (in this case, CCL19 on the right and plain medium on the left). (ii) After 180 min., a steady-state diffusion concentration gradient was reached. (iii) At this time, the cell-matrix mixture was added to the center channel, containing an average chemokine concentration of the two side channels. (iv) After several minutes, the gradient is reestablished within the center channel, before cell tracking began. (B) Temporal gradient establishment of CCL19 from experiment (solid line) and computation (dashed line). (C) Spatial gradient establishment at different time points of CCL19 (top) and CCL21 (bottom) to a collagen-MG matrix using fluorescently tagged chemokines; inserts show confocal images at 300 min. Darkest blue and red lines indicate 0 and 180 min., respectively, with 10-min. intervals shown between. (D) CCL19 (blue) and CCL21 (red) gradients at steady-state from experiment (solid lines) and computation (dashed lines).
Fig. 2.
Dendritic cells display mild chemokinesis to CCR7 ligands. (A) Percentage of migrating cells (%M); *P = 0.0366. (B) Average cell speed; *P < 0.05 and **P < 0.01. (A and B) All values were normalized to controls for each experiment, and comparisons were made using one-way ANOVA and Tukey posttest. (C, D) Histograms of normalized cell speeds in various concentration ranges of (C) CCL19 and (D) CCL21. (A_–_D) For each data point, n = 2–14, each representing the average of 71–594 cells.
Fig. 3.
Dendritic cell chemosensitivity to CCL21 is greater than to CCL19. (A) Directed migration velocity (V x) as a function of chemokine gradient (∇C). Solid lines show the fit of data to Eq. 1. (B) V x as a function of the average concentration (C_Avg). (C) V x as a function of the relative gradient (∇_C/_C_Avg). (A–C) For each data point, n = 2 - 14, each representing an average of 70–850 cells. Error bars indicate 95% confidence interval.
Fig. 4.
Dendritic cell migration is preferentially skewed towards CCL21 vs. CCL19. (A) The average velocity V x of cells in competing gradients of CCL19 and CCL21 as indicated. Dark columns: 1.5 mg/mL collagen ± 10% MG, white columns: collagen only. *P < 0.05, **P < 0.01, and ***P < 0.001 compared to group D with one-way ANOVA and Tukey posttest. Error bars show 95% confidence intervals. For each data point, n = 2, each representing an average of 84–123 cells. (B_–_E) Computationally estimated concentration gradients corresponding to the scenarios in (A). Lines indicate concentrations of CCL19 (dotted black line), soluble CCL21 (solid gray line), and total CCR7 ligand (solid black line).
Similar articles
- CCL19 with CCL21-tail displays enhanced glycosaminoglycan binding with retained chemotactic potency in dendritic cells.
Jørgensen AS, Adogamhe PE, Laufer JM, Legler DF, Veldkamp CT, Rosenkilde MM, Hjortø GM. Jørgensen AS, et al. J Leukoc Biol. 2018 Aug;104(2):401-411. doi: 10.1002/JLB.2VMA0118-008R. Epub 2018 May 16. J Leukoc Biol. 2018. PMID: 29768676 - Fluorescently Tagged CCL19 and CCL21 to Monitor CCR7 and ACKR4 Functions.
Purvanov V, Matti C, Samson GPB, Kindinger I, Legler DF. Purvanov V, et al. Int J Mol Sci. 2018 Dec 4;19(12):3876. doi: 10.3390/ijms19123876. Int J Mol Sci. 2018. PMID: 30518137 Free PMC article. - Autocrine CCL19 blocks dendritic cell migration toward weak gradients of CCL21.
Hansen M, Met Ö, Larsen NB, Rosenkilde MM, Andersen MH, Svane IM, Hjortø GM. Hansen M, et al. Cytotherapy. 2016 Sep;18(9):1187-96. doi: 10.1016/j.jcyt.2016.06.010. Epub 2016 Jul 14. Cytotherapy. 2016. PMID: 27424146 - Common and biased signaling pathways of the chemokine receptor CCR7 elicited by its ligands CCL19 and CCL21 in leukocytes.
Hauser MA, Legler DF. Hauser MA, et al. J Leukoc Biol. 2016 Jun;99(6):869-82. doi: 10.1189/jlb.2MR0815-380R. Epub 2016 Jan 4. J Leukoc Biol. 2016. PMID: 26729814 Review. - Biased signaling of G protein-coupled receptors - From a chemokine receptor CCR7 perspective.
Jørgensen AS, Rosenkilde MM, Hjortø GM. Jørgensen AS, et al. Gen Comp Endocrinol. 2018 Mar 1;258:4-14. doi: 10.1016/j.ygcen.2017.07.004. Epub 2017 Jul 8. Gen Comp Endocrinol. 2018. PMID: 28694053 Review.
Cited by
- A simple, low cost and reusable microfluidic gradient strategy and its application in modeling cancer invasion.
Samandari M, Rafiee L, Alipanah F, Sanati-Nezhad A, Javanmard SH. Samandari M, et al. Sci Rep. 2021 May 13;11(1):10310. doi: 10.1038/s41598-021-89635-0. Sci Rep. 2021. PMID: 33986379 Free PMC article. - A contact line pinning based microfluidic platform for modelling physiological flows.
Tung CK, Krupa O, Apaydin E, Liou JJ, Diaz-Santana A, Kim BJ, Wu M. Tung CK, et al. Lab Chip. 2013 Oct 7;13(19):3876-85. doi: 10.1039/c3lc50489a. Lab Chip. 2013. PMID: 23917952 Free PMC article. - Modular microfluidics for life sciences.
Wu J, Fang H, Zhang J, Yan S. Wu J, et al. J Nanobiotechnology. 2023 Mar 11;21(1):85. doi: 10.1186/s12951-023-01846-x. J Nanobiotechnology. 2023. PMID: 36906553 Free PMC article. Review. - Agarose Spot as a Comparative Method for in situ Analysis of Simultaneous Chemotactic Responses to Multiple Chemokines.
Ahmed M, Basheer HA, Ayuso JM, Ahmet D, Mazzini M, Patel R, Shnyder SD, Vinader V, Afarinkia K. Ahmed M, et al. Sci Rep. 2017 Apr 21;7(1):1075. doi: 10.1038/s41598-017-00949-4. Sci Rep. 2017. PMID: 28432337 Free PMC article. - Directing three-dimensional multicellular morphogenesis by self-organization of vascular mesenchymal cells in hyaluronic acid hydrogels.
Zhu X, Gojgini S, Chen TH, Fei P, Dong S, Ho CM, Segura T. Zhu X, et al. J Biol Eng. 2017 Apr 3;11:12. doi: 10.1186/s13036-017-0055-6. eCollection 2017. J Biol Eng. 2017. PMID: 28392831 Free PMC article.
References
- Randolph GJ, Angeli V, Swartz MA. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol. 2005;5:617–628. - PubMed
- Campbell JJ, et al. Chemokines and the arrest of lymphocytes rolling under flow conditions. Science. 1998;279:381–384. - PubMed
- Forster R, Davalos-Misslitz AC, Rot A. CCR7 and its ligands: balancing immunity and tolerance. Nat Rev Immunol. 2008;8:362–371. - PubMed
- Sullivan SK, McGrath DA, Grigoriadis D, Bacon KB. Pharmacological and signaling analysis of human chemokine receptor CCR-7 stably expressed in HEK-293 cells: high-affinity binding of recombinant ligands MIP-3beta and SLC stimulates multiple signaling cascades. Biochem Biophys Res Commun. 1999;263:685–690. - PubMed
- Ott TR, et al. The N-terminal domain of CCL21 reconstitutes high affinity binding, G protein activation, and chemotactic activity, to the C-terminal domain of CCL19. Biochem Biophys Res Commun. 2006;348:1089–1093. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources