Mechanism underlying selective regulation of G protein-gated inwardly rectifying potassium channels by the psychostimulant-sensitive sorting nexin 27 - PubMed (original) (raw)
Mechanism underlying selective regulation of G protein-gated inwardly rectifying potassium channels by the psychostimulant-sensitive sorting nexin 27
Bartosz Balana et al. Proc Natl Acad Sci U S A. 2011.
Abstract
G protein-gated inwardly rectifying potassium (GIRK) channels are important gatekeepers of neuronal excitability. The surface expression of neuronal GIRK channels is regulated by the psychostimulant-sensitive sorting nexin 27 (SNX27) protein through a class I (-X-Ser/Thr-X-Φ, where X is any residue and Φ is a hydrophobic amino acid) PDZ-binding interaction. The G protein-insensitive inward rectifier channel (IRK1) contains the same class I PDZ-binding motif but associates with a different synaptic PDZ protein, postsynaptic density protein 95 (PSD95). The mechanism by which SNX27 and PSD95 discriminate these channels was previously unclear. Using high-resolution structures coupled with biochemical and functional analyses, we identified key amino acids upstream of the channel's canonical PDZ-binding motif that associate electrostatically with a unique structural pocket in the SNX27-PDZ domain. Changing specific charged residues in the channel's carboxyl terminus or in the PDZ domain converts the selective association and functional regulation by SNX27. Elucidation of this unique interaction site between ion channels and PDZ-containing proteins could provide a therapeutic target for treating brain diseases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
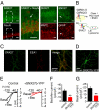
Expression of SNX27 and regulation of GIRK channels in rat hippocampal neurons. (A) Coronal sections of rat CA1 hippocampus immunostained with a pan SNX27 (SNX27a/b; green) antibody and NeuN, MAP2, or GFAP antibody (red). The short arrow highlights glial cells expressing SNX27a/b. (Inset) Zoom of CA1 pyramidal neurons for SNX27/NeuN staining; long arrow indicates a dendrite. (Scale bar: 30 μm). (B) Schematic for regulation of GIRK channels by SNX27 based on previous studies (6, 7). SNX27 reduces plasma membrane (PM) expression of GIRK channels by targeting GIRK3-containing channels via a class I PDZ motif (ESKV) in to the early endosomes (EE) and subsequent degradation in late endosomes/lysosomes. (C) Fluorescent image of cultured rat hippocampal neuron immunostained with pan SNX27 (green) and early endosomal antigen-1 antibody (red). (D) Fluorescent image of cultured rat hippocampal neuron infected with SNX27b-YFP lentivirus. (E) Current-voltage plots show inwardly rectifying K+ currents recorded from a neuron expressing GFP (control) or SNX27b-YFP. GABAB receptor-activated (Bac; 100 μM) and basal Ba2+-sensitive (1 mM) GIRK currents are shown (Kout = 20 mM) (
Table S1
). (F and G) Bar charts show mean current density, pA/pF, ± SEM. **P < 0.01 by the Student t test. n = 10–12.
Fig. 2.
Structural determination of SNX27-PDZ bound to GIRK3 peptide. (A and B) Schematics show SNX27 with a lipid-binding Phox domain (PX), a Ras-associating domain (RA), and a single class I PDZ domain (10) and PSD95 with three class I/II PDZ domains, a SH3 domain, and a GK domain (19). (A) SNX27-PDZ binds to the GST-tagged carboxyl terminus of GIRK3, but not to that of IRK1 or Kv1.4. (B) PSD95-PDZ2 and PSD95-PDZ1,2 bind to IRK1 and Kv1.4, but not to GIRK3. PDZ3 does not bind to IRK1, GIRK3, or Kv1.4. (Upper) Immunoblot with anti-H6 antibody for detecting H8-tagged probe. (Lower) Ponceau S stain for GST proteins. Probes are H8-SNX27-PDZ (500 nM) or 250 nM H8-PSD95-PDZ1,2 (250 nM). (C) Alignment of PDZ domains for rat SNX27 and PSD95. Red boxes indicate mutations in SNX27-PDZ that did not reverse binding selectivity (
Fig. S2
). (D) High-resolution (1.68 Å) crystal structure of the SNX27-PDZ-ESESKV fusion protein. A ribbon model for the PDZ domain (green) with the bound ESESKV peptide sequence. Residues 0 to −4 of the peptide are tightly bound to the βB strand and αB helix of the PDZ domain, forming an antiparallel sheet with the βB strand. E−5 faces a pocket formed by the βB strand, βC, and the βB–βC loop. (E) The canonical ligand-binding pocket of SNX27-PDZ-ESESKV. (F) The electropositive pocket (R56, R66, and R98) around the negatively charged E−5 is shown.
Fig. 3.
Critical role for charge of the −5 residue in determining PDZ binding specificity for SNX27. (A) Amino acid sequence for GIRK3 and IRK1 C-terminal end (−3 to 0 residues) highlighted for the class I motif. An in vitro binding assay showed that converting E-1I0 to K-1V0 in IRK1 (IRK1*) does not create binding to SNX27-PDZ. (B) In vitro protein–protein binding assay with single or double mutations of the −5 and −4 positions in GIRK3 and IRK1*. (C and D) Effect of systematic substitutions at R−5 in IRK1* (Lys, Asp, Gln, Ala, or Trp) on binding to H8-SNX27-PDZ or H8-PSD95-PDZ1,2. (D) Bar graphs show quantification of binding to IRK1* mutants. SNX27-PDZ binding prefers ligands with acidic or neutral but not basic amino acids at the −5 position, whereas PSD95-PDZ1,2 prefers basic or neutral residues, but not acidic residues, at −5. Relative binding was normalized to GST-IRK1 and H8-PSD95-PDZ1,2 or to GST-GIRK3 and H8-SNX27-PDZ. Values are mean ± SEM. n = 3–5 experiments. **P < 0.05 vs. WT channels.
Fig. 4.
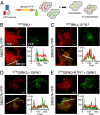
Mutation of −5 and −4 residues reverses association and functional regulation of IRK1 and GIRK1/3 channels by SNX27b in COS7. (A) Cartoon illustrates the colocalization assay for CFP-tagged channel and YFP-tagged SNX27b using TIRF microscopy. If SNX27b and channel appear as overlapping puncta, then the two proteins colocalize. CFP is pseudo-colored red, and YFP is green. (B) SNX27b-YFP does not colocalize with WT CFPIRK1*. (C) In contrast, CFPIRK1*-E-5S−4 does colocalize with SNX27b. (D) Colocalization of CFPGIRK3 with SNX27b-YFP. (E) Colocalization is disrupted with the R−5R−4 mutation (CFPGIRK3-R−5R−4). Graphs show fluorescence intensity for the line profile (white line in merged image). GIRK1 was coexpressed with CFPGIRK3 and CFPGIRK3-R−5R−4 to promote surface expression. (Scale bar: 10 μm.)
Fig. 5.
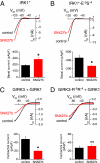
Mutation of the −5 and −4 positions alters functional regulation of inwardly rectifying currents by SNX27b expressed in HEK293T cells. (A and B) Current-voltage plots shows examples of inwardly rectifying K+ currents for IRK1* (A) and IRK1*-E−5S−4 (B) channels in the absence or presence of SNX27b (Kout = 5 mM). Bar graphs show mean values ± SEM (n = 11–14). SNX27b reduces the amplitude of Ba2+-sensitive IRK1*-E−5S−4 currents while increasing the amplitude of IRK1* currents (see text). (C and D) Current-voltage plots show the effect of SNX27b on M2 muscarinic receptor-activated (carbachol-induced) currents through GIRK1/GIRK3 (C) or GIRK1/GIRK3-R−5R−4 (D) channels (Kout = 20 mM). Bar graphs show mean induced currents ± SEM (n = 8–15) (
Table S1
). *P < 0.05, Student t test.
Fig. 6.
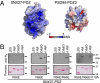
Two sites involved in PDZ binding selectivity. A role for electrostatic potential of the βB–βC loop in determining SNX27 PDZ specificity. (A) Surface charge was mapped onto the molecular surface of SNX27-PDZ and PSD95-PDZ2 (26), with superimposed GIRK3 (-ESESKV) and IRK1 (-RRESEI) peptides, respectively. The negative E−5 of GIRK3 faces an electropositive groove between βB and the βB–βC loop of SNX27-PDZ. The positive R−5 and R−4 of IRK1 interact with negative charges near the αB helix of PSD95-PDZ2 (26). (B) SNX27 R56E eliminates binding, whereas an SNX27 R66E shows weak binding to IRK1* and GIRK3. SNX27 R56E/R66E creates selective binding to IRK1* and no detectable binding to GIRK3. Combining the I119A mutation in the 0 position with R56E/R66E converts SNX27-PDZ binding from GIRK3 to IRK1.
Similar articles
- Sorting nexin 27 regulation of G protein-gated inwardly rectifying K⁺ channels attenuates in vivo cocaine response.
Munoz MB, Slesinger PA. Munoz MB, et al. Neuron. 2014 May 7;82(3):659-69. doi: 10.1016/j.neuron.2014.03.011. Neuron. 2014. PMID: 24811384 Free PMC article. - A unique sorting nexin regulates trafficking of potassium channels via a PDZ domain interaction.
Lunn ML, Nassirpour R, Arrabit C, Tan J, McLeod I, Arias CM, Sawchenko PE, Yates JR 3rd, Slesinger PA. Lunn ML, et al. Nat Neurosci. 2007 Oct;10(10):1249-59. doi: 10.1038/nn1953. Epub 2007 Sep 2. Nat Neurosci. 2007. PMID: 17828261 - Subunit-specific regulation of Kir3 channels by sorting nexin 27.
Nassirpour R, Slesinger PA. Nassirpour R, et al. Channels (Austin). 2007 Sep-Oct;1(5):331-3. doi: 10.4161/chan.5191. Epub 2007 Oct 20. Channels (Austin). 2007. PMID: 18690037 - Structural Insights into GIRK Channel Function.
Glaaser IW, Slesinger PA. Glaaser IW, et al. Int Rev Neurobiol. 2015;123:117-60. doi: 10.1016/bs.irn.2015.05.014. Epub 2015 Jun 22. Int Rev Neurobiol. 2015. PMID: 26422984 Review. - Emerging roles for G protein-gated inwardly rectifying potassium (GIRK) channels in health and disease.
Lüscher C, Slesinger PA. Lüscher C, et al. Nat Rev Neurosci. 2010 May;11(5):301-15. doi: 10.1038/nrn2834. Epub 2010 Apr 14. Nat Rev Neurosci. 2010. PMID: 20389305 Free PMC article. Review.
Cited by
- Plasticity of PDZ domains in ligand recognition and signaling.
Ivarsson Y. Ivarsson Y. FEBS Lett. 2012 Aug 14;586(17):2638-47. doi: 10.1016/j.febslet.2012.04.015. Epub 2012 Apr 21. FEBS Lett. 2012. PMID: 22576124 Free PMC article. Review. - GIRK3 gates activation of the mesolimbic dopaminergic pathway by ethanol.
Herman MA, Sidhu H, Stouffer DG, Kreifeldt M, Le D, Cates-Gatto C, Munoz MB, Roberts AJ, Parsons LH, Roberto M, Wickman K, Slesinger PA, Contet C. Herman MA, et al. Proc Natl Acad Sci U S A. 2015 Jun 2;112(22):7091-6. doi: 10.1073/pnas.1416146112. Epub 2015 May 11. Proc Natl Acad Sci U S A. 2015. PMID: 25964320 Free PMC article. - Additive energetic contributions of multiple peptide positions determine the relative promiscuity of viral and human sequences for PDZ domain targets.
Tahti EF, Blount JM, Jackson SN, Gao M, Gill NP, Smith SN, Pederson NJ, Rumph SN, Struyvenberg SA, Mackley IGP, Madden DR, Amacher JF. Tahti EF, et al. Protein Sci. 2023 Apr;32(4):e4611. doi: 10.1002/pro.4611. Protein Sci. 2023. PMID: 36851847 Free PMC article. - Snx14 regulates neuronal excitability, promotes synaptic transmission, and is imprinted in the brain of mice.
Huang HS, Yoon BJ, Brooks S, Bakal R, Berrios J, Larsen RS, Wallace ML, Han JE, Chung EH, Zylka MJ, Philpot BD. Huang HS, et al. PLoS One. 2014 May 23;9(5):e98383. doi: 10.1371/journal.pone.0098383. eCollection 2014. PLoS One. 2014. PMID: 24859318 Free PMC article. - SNX16 negatively regulates the migration and tumorigenesis of MCF-7 cells.
Zhang L, Qin D, Hao C, Shu X, Pei D. Zhang L, et al. Cell Regen. 2013 Apr 30;2(1):3. doi: 10.1186/2045-9769-2-3. eCollection 2013. Cell Regen. 2013. PMID: 25408875 Free PMC article.
References
- Reuveny E, et al. Activation of the cloned muscarinic potassium channel by G protein βγ subunits. Nature. 1994;370:143–146. - PubMed
- Wickman KD, et al. Recombinant G-protein βγ-subunits activate the muscarinic-gated atrial potassium channel. Nature. 1994;368:255–257. - PubMed
- Huang CS, et al. Common molecular pathways mediate long-term potentiation of synaptic excitation and slow synaptic inhibition. Cell. 2005;123:105–118. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DA019022/DA/NIDA NIH HHS/United States
- R01 DA019022/DA/NIDA NIH HHS/United States
- R01 NS037682/NS/NINDS NIH HHS/United States
- GM521223/GM/NIGMS NIH HHS/United States
- NS37682/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases