Roles of the Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR pathways in controlling growth and sensitivity to therapy-implications for cancer and aging - PubMed (original) (raw)
Review
doi: 10.18632/aging.100296.
William H Chappell, Stephen L Abrams, Ruth C Kempf, Jacquelyn Long, Piotr Laidler, Sanja Mijatovic, Danijela Maksimovic-Ivanic, Franca Stivala, Maria C Mazzarino, Marco Donia, Paolo Fagone, Graziella Malaponte, Ferdinando Nicoletti, Massimo Libra, Michele Milella, Agostino Tafuri, Antonio Bonati, Jörg Bäsecke, Lucio Cocco, Camilla Evangelisti, Alberto M Martelli, Giuseppe Montalto, Melchiorre Cervello, James A McCubrey
Affiliations
- PMID: 21422497
- PMCID: PMC3091517
- DOI: 10.18632/aging.100296
Review
Roles of the Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR pathways in controlling growth and sensitivity to therapy-implications for cancer and aging
Linda S Steelman et al. Aging (Albany NY). 2011 Mar.
Abstract
Dysregulated signaling through the Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR pathways is often the result of genetic alterations in critical components in these pathways or upstream activators. Unrestricted cellular proliferation and decreased sensitivity to apoptotic-inducing agents are typically associated with activation of these pro-survival pathways. This review discusses the functions these pathways have in normal and neoplastic tissue growth and how they contribute to resistance to apoptotic stimuli. Crosstalk and commonly identified mutations that occur within these pathways that contribute to abnormal activation and cancer growth will also be addressed. Finally the recently described roles of these pathways in cancer stem cells, cellular senescence and aging will be evaluated. Controlling the expression of these pathways could ameliorate human health.
Figures
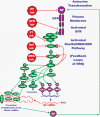
Figure 1.. Overview of the Ras/Raf/MEK/ERK Pathway and Potential Sites of Therapeutic Intervention with Small Molecule Membrane-Permeable Inhibitors
The Ras/Raf/MEK/ERK pathway is regulated by Ras (indicated in green ovals), as well as various upstream growth factor receptors (indicated in purple) and non-receptor kinases. Sites where various small molecule inhibitors suppress this pathway are indicated by red octagons. The downstream transcription factors regulated by this pathway are indicated in purple diamond shaped outlines. The Ras/Raf/MEK/ERK pathway also interacts with key proteins involved in protein translation (indicated in green ovals). The Ras/Raf/MEK/ERK pathway aids in the assembly of the protein translation complex responsible for the translation of “weak” mRNAs (indicated in a red line folding over on itself) important in the prevention of apoptosis. This drawing depicts a relative common, yet frequently overlooked phenomenon in human cancer, autocrine transformation. GF = growth factor, GFR = growth factor receptor.
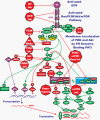
Figure 2.. Overview of the Ras/PI3K/PTEN/Akt/mTOR Pathway and Potential Sites of Therapeutic Intervention
The Ras/PI3K/PTEN/mTOR pathway is regulated by Ras (indicted in green ovals), as well as various upstream growth factor receptors (indicated in purple). Sites where various small molecule inhibitors suppress this pathway are indicated by red octagons. Naturally occurring miRNAs have been discovered to certain components of this pathway (e.g., PTEN) and are indicated in a red triangle; other miRNAs to other components, especially tumor suppressor genes will likely be discovered. The downstream transcription factors regulated by this pathway are indicated in diamond shaped purple (active) or red (inactivated) outlines. This drawing depicts some of the complicated regulations of this pathway by both positive and negative phosphorylation events which serve to fine tune this pathway. Phosphorylation of some molecules by certain kinases (e.g., phosphorylation of β-catenin by glycogen synthase kinase-3β [GSK-3β], indicated in red oval) results in their proteosomal degradation (indicated in red box), while phosphorylation of some molecules by certain kinases (e.g., β-catenin by Akt) results in their activation (nuclear translocation, indicated in green box). The Ras/PI3K/PTEN/Akt/mTOR pathway plays a key role in regulating p53 activity (indicated in purple diamond) by phosphorylating MDM2 (indicated in red oval) which controls the stability of p53 by ubiquitination. The Ras/PI3K/PTEN/Akt/mTOR pathway plays a key role in regulating critical proteins involved in protein translation (indicated in green ovals), especially those necessary for the translation of “weak” mRNAs (mTORC1, grouped together a purple box). This pathway also indicates that Akt can result in the activation of downstream mTOR which can subsequently serve as either a negative feed back to inactivate Akt by p70S6K or activate Akt by mTORC2 (grouped together in a blue box). GF = growth factor, GFR = growth factor receptor.
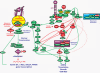
Figure 3.. Interactions between the Ras/Raf/MEK/ERK, Ras/PI3K/PTEN/mTOR and Wnt/β-Catenin Pathways that Result in the Regulation of Protein Translation and Gene Transcription
The Ras/Raf/MEK/ERK and Ras/PI3K/PTEN/Akt/mTOR pathways can affect protein translation by complex interactions regulating the mTORC1 (grouped together in a purple box) and mTORC2 (grouped together in a blue box) complexes. GF stimulation results in GFR activation which can activate both the Ras/Raf/MEK/ERK and Ras/PI3K/PTEN/Akt/mTOR pathways. Akt can phosphorylate and inhibit the effects of GSK-3β, TSC2 and PRAS-40 (indicated in red ovals), which result in mTORC1 activation. ERK and PDK1 can phosphorylate p90Rsk1 (indicated in green ovals), which in turn can phosphorylate and inhibit TSC2 (indicated in red oval). Akt-mediated phosphorylation of GSK-3β also affects the Wnt/β-catenin pathway and EMT. Rapamycin targets mTORC1 and inhibits its activity and also results in inhibition of downstream p70S6K. The effects of rapamycin are complex as long term administration of rapamycin may prevent mTOR from associating with mTORC2 and hence full activation of Akt is prevented. However, rapamycin treatment may result in activation of PI3K, by inhibiting the effects of p70S6K on IRS-1 phosphorylation which results in PI3K and Akt activation. Also rapamycin treatment may result in the activation of ERK in some cells, presumably by inhibition of the p70S6K mediated inhibition of IRS1. These later two effects of rapamycin could have positive effects on cell growth. Energy deprivation will result in the activation of
s
erine/
t
hreonine
k
inase 11 (STK11 a.k.a LKB1) and
AMPk
inase (AMPK) which can result in TSC2 activation (indicated in red ovals) and subsequent suppression of mTORC1. In contrast Akt can phosphorylate and inhibit the activity of AMPK. Inhibition of PDK-1 activity can also result in activation of mTORC1, presumably by suppression of p70S6K and hence inhibition of IRS1 (indicated in red oval) effects on PI3K activity. The PTEN, TSC1, TSC2 and LKB1 tumor suppressor genes all converge on the mTORC1 complex to regulate protein translation. Thus the Ras/Raf/MEK/ERK and Ras/PI3K/PTEN/Akt/mTOR pathways can finely tune protein translation and cell growth by regulating mTORC1. Rapamycin can have diverse effects on these processes. Also these pathways can interact with the Wnt/β-catenin pathway which is important in developmental processes, EMT and CICs. Upon activation of the Wnt pathway, β-catenin forms a complex with Bcl-9, PYGO, plakoglobulin and TCF/LEF which result in the transcription of critical genes including cyclin D1, c-Myc, SALL4 and PPARδ.
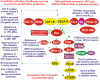
Figure 4.. Dysregulated Expression of Upstream Receptors and Kinases Can Result in Activation of the Ras/Raf/MEK/ERK and Ras/PI3K/PTEN/Akt/mTOR Pathways
Sometimes dysregulated expression of growth factor receptors occurs by either increased expression or genomic amplifications (e.g., VEGFR, EGFR, HER2, IGF1R). Mutations have been detected in EGFR, FLT3, KIT, PDGFR, PIK3CA, RAS, BRAF, MEK1/MEK2, SOS, PTPN11 (indicated in red ovals), and PTEN (indicated in a purple square) . Akt and Rheb are overexpressed in certain cancers. Other signaling molecules which may be overexpressed (e.g., IGF-1R, VEGF-R, ERK, mTOR, p70S6K) but not necessarily mutated or amplified are indicated in yellow ovals. The MDM2 ubiquitin ligase is indicated in a green oval. The p53 tumor suppressor is one of the most frequently inactivated genes in human cancer and has multiple effects on these pathways and is indicated in a purple oval. Amplifications of HER2 and EGFR are detected in certain cancer types. The BCRABL chromosomal translocation is present in virtually all
c
hronic
m
yeloid
l
eukemias (CMLs) and some
a
cute
l
ymphatic
l
eukemias (ALLs). Many of these mutations and chromosomal translocations result in the activation of the Ras/Raf/MEK/ERK and Ras/PI3K/PTEN/Akt/mTOR cascades. These pathways can also be activated by autocrine growth stimulation, the genetic basis of which is frequently unknown. Deregulated expression of these pathways can result in cancer as well as premature aging.
Similar articles
- Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR inhibitors: rationale and importance to inhibiting these pathways in human health.
Chappell WH, Steelman LS, Long JM, Kempf RC, Abrams SL, Franklin RA, Bäsecke J, Stivala F, Donia M, Fagone P, Malaponte G, Mazzarino MC, Nicoletti F, Libra M, Maksimovic-Ivanic D, Mijatovic S, Montalto G, Cervello M, Laidler P, Milella M, Tafuri A, Bonati A, Evangelisti C, Cocco L, Martelli AM, McCubrey JA. Chappell WH, et al. Oncotarget. 2011 Mar;2(3):135-64. doi: 10.18632/oncotarget.240. Oncotarget. 2011. PMID: 21411864 Free PMC article. Review. - Therapeutic resistance resulting from mutations in Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR signaling pathways.
McCubrey JA, Steelman LS, Kempf CR, Chappell WH, Abrams SL, Stivala F, Malaponte G, Nicoletti F, Libra M, Bäsecke J, Maksimovic-Ivanic D, Mijatovic S, Montalto G, Cervello M, Cocco L, Martelli AM. McCubrey JA, et al. J Cell Physiol. 2011 Nov;226(11):2762-81. doi: 10.1002/jcp.22647. J Cell Physiol. 2011. PMID: 21302297 Review. - Roles of the RAF/MEK/ERK and PI3K/PTEN/AKT pathways in malignant transformation and drug resistance.
McCubrey JA, Steelman LS, Abrams SL, Lee JT, Chang F, Bertrand FE, Navolanic PM, Terrian DM, Franklin RA, D'Assoro AB, Salisbury JL, Mazzarino MC, Stivala F, Libra M. McCubrey JA, et al. Adv Enzyme Regul. 2006;46:249-79. doi: 10.1016/j.advenzreg.2006.01.004. Epub 2006 Jul 18. Adv Enzyme Regul. 2006. PMID: 16854453 - Mutations and deregulation of Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR cascades which alter therapy response.
McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Montalto G, Cervello M, Nicoletti F, Fagone P, Malaponte G, Mazzarino MC, Candido S, Libra M, Bäsecke J, Mijatovic S, Maksimovic-Ivanic D, Milella M, Tafuri A, Cocco L, Evangelisti C, Chiarini F, Martelli AM. McCubrey JA, et al. Oncotarget. 2012 Sep;3(9):954-87. doi: 10.18632/oncotarget.652. Oncotarget. 2012. PMID: 23006971 Free PMC article. Review. - PI3K/Akt/mTOR and Ras/Raf/MEK/ERK signaling pathways inhibitors as anticancer agents: Structural and pharmacological perspectives.
Asati V, Mahapatra DK, Bharti SK. Asati V, et al. Eur J Med Chem. 2016 Feb 15;109:314-41. doi: 10.1016/j.ejmech.2016.01.012. Epub 2016 Jan 12. Eur J Med Chem. 2016. PMID: 26807863 Review.
Cited by
- Autophagy-related gene 12 (ATG12) is a novel determinant of primary resistance to HER2-targeted therapies: utility of transcriptome analysis of the autophagy interactome to guide breast cancer treatment.
Cufí S, Vazquez-Martin A, Oliveras-Ferraros C, Corominas-Faja B, Urruticoechea A, Martin-Castillo B, Menendez JA. Cufí S, et al. Oncotarget. 2012 Dec;3(12):1600-14. doi: 10.18632/oncotarget.742. Oncotarget. 2012. PMID: 23307622 Free PMC article. - Oncogenic mutations of thyroid hormone receptor β.
Park JW, Zhao L, Willingham M, Cheng SY. Park JW, et al. Oncotarget. 2015 Apr 10;6(10):8115-31. doi: 10.18632/oncotarget.3466. Oncotarget. 2015. PMID: 25924236 Free PMC article. - Effects of ectopic expression of NGAL on doxorubicin sensitivity.
Chappell WH, Abrams SL, Montalto G, Cervello M, Martelli AM, Candido S, Libra M, Polesel J, Talamini R, Arlinghaus R, Steelman LS, McCubrey JA. Chappell WH, et al. Oncotarget. 2012 Oct;3(10):1236-45. doi: 10.18632/oncotarget.691. Oncotarget. 2012. PMID: 23100449 Free PMC article. - PTEN negatively regulates mTORC2 formation and signaling in grade IV glioma via Rictor hyperphosphorylation at Thr1135 and direct the mode of action of an mTORC1/2 inhibitor.
Bhattacharya K, Maiti S, Mandal C. Bhattacharya K, et al. Oncogenesis. 2016 May 30;5(5):e227. doi: 10.1038/oncsis.2016.34. Oncogenesis. 2016. PMID: 27239959 Free PMC article. - Targeting filamin A reduces K-RAS-induced lung adenocarcinomas and endothelial response to tumor growth in mice.
Nallapalli RK, Ibrahim MX, Zhou AX, Bandaru S, Sunkara SN, Redfors B, Pazooki D, Zhang Y, Borén J, Cao Y, Bergo MO, Akyürek LM. Nallapalli RK, et al. Mol Cancer. 2012 Aug 2;11:50. doi: 10.1186/1476-4598-11-50. Mol Cancer. 2012. PMID: 22857000 Free PMC article.
References
- Casar B, Pinto A, Crespo P. ERK dimmers and scaffold proteins: unexpected partners for a forgotten task. Cell Cycle. 2009;8:1007–1013. - PubMed
- McCubrey JA, Steelman LS, Abrams SL, Bertrand FE, Ludwig DE, Basecke J, et al. Targeting Survival Cascades Induced by Activation of Raf/Raf/MEK/ERK, PI3K/PTEN/Akt/mTOR and Jak/STAT pathways for effective leukemia therapy. Leukemia. 2008;22:708–722. - PubMed
- McCubrey JA, Steelman LS, Abrams SL, Chappell WH, Russo S, Ove R, et al. Emerging Raf Inhibitors. Exp Opin Emerging Drugs. 2009;14:633–648. - PubMed
- Martelli AM, Evangelisti C, Chiarini F, Grimaldi C, Cappellini A, Ognibene A, McCubrey JA. The emerging role of the phosphatiylinositol 3-kinase/Akt/mammalian target of rapamycin signaling network in normal myelopoiesis and leukemogensis. Biochim Biophys Act. 2010;1803:991–1002. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous