Microbial induction of immunity, inflammation, and cancer - PubMed (original) (raw)
Microbial induction of immunity, inflammation, and cancer
Julia B Greer et al. Front Physiol. 2011.
Abstract
The human microbiota presents a highly active metabolic that influences the state of health of our gastrointestinal tracts as well as our susceptibility to disease. Although much of our initial microbiota is adopted from our mothers, its final composition and diversity is determined by environmental factors. Westernization has significantly altered our microbial function. Extensive experimental and clinical evidence indicates that the westernized diet, rich in animal products and low in complex carbohydrates, plus the overuse of antibiotics and underuse of breastfeeding, leads to a heightened inflammatory potential of the microbiota. Chronic inflammation leads to the expression of certain diseases in genetically predisposed individuals. Antibiotics and a "clean" environment, termed the "hygiene hypothesis," has been linked to the rise in allergy and inflammatory bowel disease, due to impaired beneficial bacterial exposure and education of the gut immune system, which comprises the largest immune organ within the body. The elevated risk of colon cancer is associated with the suppression of microbial fermentation and butyrate production, as butyrate provides fuel for the mucosa and is anti-inflammatory and anti-proliferative. This article will summarize the work to date highlighting the complicated and dynamic relationship between the gut microbiota and immunity, inflammation and carcinogenesis.
Keywords: allergy; colon cancer; diet; inflammatory bowel disease; microbiota.
Figures
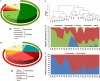
Figure 1
16S rRNA gene surveys reveal a clear separation of two children populations investigated. (A,B) Pie charts of median values of bacterial genera present in fecal samples of Burkina Faso (BF) and European Union (EU) children (>3%) found by RDP classifier v. 2.1. Rings represent corresponding phylum (Bacteroidetes in green and Firmicutes in red) for each of the most frequently represented genera. (C) Dendrogram obtained with complete linkage hierarchical clustering of the samples from BF and EU populations based on their genera. The subcluster located in the middle of the tree contains samples taken from the three youngest (1–2 years old) children of the BF group (16BF, 3BF, and 4BF) and two 1-year-old children of the EU group (2EU and 3EU). (D) Relative abundances (percentage of sequences) of the four most abundant bacterial phyla in each individual among the BF and EU children. Blue area in middle shows abundance of Actinobacteria, mainly represented by Bifidobacterium genus, in the five youngest EU and BF children. (E) Relative abundance (percentage of sequences) of Gram-negative and Gram-positive bacteria in each individual. Different distributions of Gram-negative and Gram-positive in the BF and EU populations reflect differences in the two most represented phyla, Bacteroidetes and Firmicutes. Adapted from De Filippo et al. (2010).
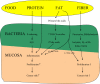
Figure A1
The relationship between macronutrients and fiber with enteric bacteria and the intestinal mucosa.
Similar articles
- The Roles of Inflammation, Nutrient Availability and the Commensal Microbiota in Enteric Pathogen Infection.
Stecher B. Stecher B. Microbiol Spectr. 2015 Jun;3(3). doi: 10.1128/microbiolspec.MBP-0008-2014. Microbiol Spectr. 2015. PMID: 26185088 - Dismicrobism in inflammatory bowel disease and colorectal cancer: changes in response of colocytes.
Tomasello G, Tralongo P, Damiani P, Sinagra E, Di Trapani B, Zeenny MN, Hussein IH, Jurjus A, Leone A. Tomasello G, et al. World J Gastroenterol. 2014 Dec 28;20(48):18121-30. doi: 10.3748/wjg.v20.i48.18121. World J Gastroenterol. 2014. PMID: 25561781 Free PMC article. Review. - Prebiotic effects: metabolic and health benefits.
Roberfroid M, Gibson GR, Hoyles L, McCartney AL, Rastall R, Rowland I, Wolvers D, Watzl B, Szajewska H, Stahl B, Guarner F, Respondek F, Whelan K, Coxam V, Davicco MJ, Léotoing L, Wittrant Y, Delzenne NM, Cani PD, Neyrinck AM, Meheust A. Roberfroid M, et al. Br J Nutr. 2010 Aug;104 Suppl 2:S1-63. doi: 10.1017/S0007114510003363. Br J Nutr. 2010. PMID: 20920376 Review. - Intestinal microbiota in inflammatory bowel disease: friend of foe?
Fava F, Danese S. Fava F, et al. World J Gastroenterol. 2011 Feb 7;17(5):557-66. doi: 10.3748/wjg.v17.i5.557. World J Gastroenterol. 2011. PMID: 21350704 Free PMC article. - Linking the Gut Microbial Ecosystem with the Environment: Does Gut Health Depend on Where We Live?
Tasnim N, Abulizi N, Pither J, Hart MM, Gibson DL. Tasnim N, et al. Front Microbiol. 2017 Oct 6;8:1935. doi: 10.3389/fmicb.2017.01935. eCollection 2017. Front Microbiol. 2017. PMID: 29056933 Free PMC article.
Cited by
- Fat, fibre and cancer risk in African Americans and rural Africans.
O'Keefe SJ, Li JV, Lahti L, Ou J, Carbonero F, Mohammed K, Posma JM, Kinross J, Wahl E, Ruder E, Vipperla K, Naidoo V, Mtshali L, Tims S, Puylaert PG, DeLany J, Krasinskas A, Benefiel AC, Kaseb HO, Newton K, Nicholson JK, de Vos WM, Gaskins HR, Zoetendal EG. O'Keefe SJ, et al. Nat Commun. 2015 Apr 28;6:6342. doi: 10.1038/ncomms7342. Nat Commun. 2015. PMID: 25919227 Free PMC article. - Brain-gut-microbe communication in health and disease.
Grenham S, Clarke G, Cryan JF, Dinan TG. Grenham S, et al. Front Physiol. 2011 Dec 7;2:94. doi: 10.3389/fphys.2011.00094. eCollection 2011. Front Physiol. 2011. PMID: 22162969 Free PMC article. - Gut Microbiota Metabolism and Interaction with Food Components.
Vernocchi P, Del Chierico F, Putignani L. Vernocchi P, et al. Int J Mol Sci. 2020 May 23;21(10):3688. doi: 10.3390/ijms21103688. Int J Mol Sci. 2020. PMID: 32456257 Free PMC article. Review. - Shaping functional gut microbiota using dietary bioactives to reduce colon cancer risk.
Seidel DV, Azcárate-Peril MA, Chapkin RS, Turner ND. Seidel DV, et al. Semin Cancer Biol. 2017 Oct;46:191-204. doi: 10.1016/j.semcancer.2017.06.009. Epub 2017 Jul 1. Semin Cancer Biol. 2017. PMID: 28676459 Free PMC article. Review. - Diet, microbiota, and microbial metabolites in colon cancer risk in rural Africans and African Americans.
Ou J, Carbonero F, Zoetendal EG, DeLany JP, Wang M, Newton K, Gaskins HR, O'Keefe SJ. Ou J, et al. Am J Clin Nutr. 2013 Jul;98(1):111-20. doi: 10.3945/ajcn.112.056689. Epub 2013 May 29. Am J Clin Nutr. 2013. PMID: 23719549 Free PMC article.
References
- Ananthakrishnan A. N., Issa M., Binion D. G. (2009). Clostridium difficile and inflammatory bowel disease. Gastroenterol. Clin. North Am. 38, 711–728 - PubMed
- Angulo S., Llopis M., Antolin M., Gironella M., Sans M., Malagelada J. R., Pique J. M., Guarner F., Panes J. (2006). Lactobacillus casei prevents the upregulation of ICAM-1 expression and leukocyte recruitment in experimental colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 291, G1155–G1162. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources