Synaptic vesicle pools: an update - PubMed (original) (raw)
Synaptic vesicle pools: an update
Annette Denker et al. Front Synaptic Neurosci. 2010.
Abstract
During the last few decades synaptic vesicles have been assigned to a variety of functional and morphological classes or "pools". We have argued in the past (Rizzoli and Betz, 2005) that synaptic activity in several preparations is accounted for by the function of three vesicle pools: the readily releasable pool (docked at active zones and ready to go upon stimulation), the recycling pool (scattered throughout the nerve terminals and recycling upon moderate stimulation), and finally the reserve pool (occupying most of the vesicle clusters and only recycling upon strong stimulation). We discuss here the advancements in the vesicle pool field which took place in the ensuing years, focusing on the behavior of different pools under both strong stimulation and physiological activity. Several new findings have enhanced the three-pool model, with, for example, the disparity between recycling and reserve vesicles being underlined by the observation that the former are mobile, while the latter are "fixed". Finally, a number of altogether new concepts have also evolved such as the current controversy on the identity of the spontaneously recycling vesicle pool.
Keywords: spontaneous release; super-pool; surface pool; synaptic vesicle pools; vesicle mobility; vesicle recycling.
Figures
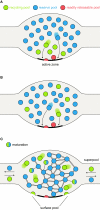
Figure 1
Synaptic vesicle pool models. (A) The classical model of three distinctly localized synaptic vesicle pools. The readily releasable pool (RRP; depicted in red) consists of the vesicles docked at the active zone and primed for release. After depletion of the RRP, the recycling pool vesicles come into play (green): these vesicles were thought to be located directly behind the RRP. Under moderate stimulation conditions, they are recruited to the active zone (left arrow) and released. Very high stimulation causes the depletion of the recycling pool and recruits the reserve pool vesicles (blue) from areas even further away from the active zone (right arrow). (B) A pool model taking into account the spatial intermixing of vesicles (Rizzoli and Betz, 2005). In contrast to the previous model, in which localization within the nerve terminal determines release kinetics and consequently pool affiliation, here the recycling and reserve pool vesicles are thought to be spatially (but not functionally) intermixed. Upon arrival of an action potential, RRP vesicles (which are in this model only the “lucky” recycling pool vesicles finding themselves docked and primed at the active zone) are released first, followed by release of recycling pool vesicles (right arrow). As above, continuous stimulation at high-frequency results in recycling pool depletion and recruitment of reserve pool vesicles (left arrow). (C) A new pool model implementing recent findings. As above, recycling and reserve pool vesicles are thought to be spatially intermixed, but display different mobilities: recycling pool vesicles are highly mobile; the movement of reserve pool vesicles is restricted by binding to some scaffolding molecule. With time, recycling pool vesicles can “mature” into reserve pool vesicles, by binding the scaffolding molecule(s) and integrating into the vesicle cluster, as indicated by the green-blue intermediate forms. These are not connected to the cluster as tightly as the reserve pool vesicles (indicated by single or double bonds). Recycling pool vesicles reach the active zone, due to their permanent mobility; stimulation does not “move” them toward the active zone, it just allows them to fuse. Furthermore, the surface pool of fused vesicles is indicated; they would be endocytosed to form part of the recycling pool (see main text). The frequent exchange of both recycling and reserve vesicles between synapses forms what has been termed a super-pool (see main text).
Figure 2
Vesicle release under different stimulation conditions. (A) Unphysiological stimulation results in release kinetics in accordance with the three pool model (depicted here for spatially intermixed recycling and reserve pool vesicles, Figures 1B,C). Upon arrival of an action potential (AP), the vesicles of the RRP (red) are released immediately. Further stimulation results in the recruitment and release of recycling pool vesicles (green), with release kinetics slower than for the RRP (as the recycling pool vesicles still need to reach the active zone and undergo docking and fusion before exocytosis). When high-frequency stimulation continues, the reserve pool vesicles are finally released (blue), displaying even slower release kinetics than the recycling pool. Therefore, a tri-phasic exocytosis process is observed. When the stimulation has ceased, vesicle components are retrieved from the plasma membrane and the three vesicle pools are reformed (vacant RRP positions are refilled as some recycling pool vesicles dock at the active zone). The lower panels indicate summed release. (B) Physiological stimulation does not allow for the distinction of separate vesicle pools. Upon arrival of an action potential, some of the (RRP) vesicles docked at the active zone are released. However, the mild stimulation paradigm employed allows for a recovery phase, during which these vesicles are retrieved as recycling pool vesicles. They can then either transform into reserve pool vesicles (indicated by the green-blue gradient) or maintain their recycling pool status. Repeated action potentials cause further release of vesicles docked at the active zone. Occasionally, a reserve pool vesicle docked at the active zone will also undergo fusion with the plasma membrane. Upon endocytosis, it will replenish the recycling pool. As no differences in release kinetics can be observed, distinct vesicle pools are not evident from the summed release (indicated by the lower panels). Note that it is also possible that the reserve pool vesicles join the recycling pool before fusion, rather than fusing and becoming recycling vesicles only after endocytosis (not indicated in the graphs); there is currently no evidence for this model that we are aware of (see main text for details).
Similar articles
- [Peculiarities of synaptic vesicle recycling in frog and mouse motor nerve terminals].
Zefirov AL, Zakharov AV, Mukhamedzianov RD, Petrov AM. Zefirov AL, et al. Zh Evol Biokhim Fiziol. 2008 Nov-Dec;44(6):603-12. Zh Evol Biokhim Fiziol. 2008. PMID: 19198161 Russian. - Complexin Mutants Reveal Partial Segregation between Recycling Pathways That Drive Evoked and Spontaneous Neurotransmission.
Sabeva N, Cho RW, Vasin A, Gonzalez A, Littleton JT, Bykhovskaia M. Sabeva N, et al. J Neurosci. 2017 Jan 11;37(2):383-396. doi: 10.1523/JNEUROSCI.1854-16.2016. J Neurosci. 2017. PMID: 28077717 Free PMC article. - Synapsin regulation of vesicle organization and functional pools.
Bykhovskaia M. Bykhovskaia M. Semin Cell Dev Biol. 2011 Jun;22(4):387-92. doi: 10.1016/j.semcdb.2011.07.003. Epub 2011 Jul 31. Semin Cell Dev Biol. 2011. PMID: 21827866 Review. - Revisiting synaptic vesicle pool localization in the Drosophila neuromuscular junction.
Denker A, Kröhnert K, Rizzoli SO. Denker A, et al. J Physiol. 2009 Jun 15;587(Pt 12):2919-26. doi: 10.1113/jphysiol.2009.170985. Epub 2009 Apr 29. J Physiol. 2009. PMID: 19403600 Free PMC article. - Monitoring synaptic vesicle recycling in frog motor nerve terminals with FM dyes.
Rizzoli SO, Richards DA, Betz WJ. Rizzoli SO, et al. J Neurocytol. 2003 Jun-Sep;32(5-8):539-49. doi: 10.1023/B:NEUR.0000020609.19873.e8. J Neurocytol. 2003. PMID: 15034252 Review.
Cited by
- Actomyosin-mediated inhibition of synaptic vesicle release under CB1R activation.
McFadden MH, Emeritt MB, Xu H, Cui Y, Leterrier C, Zala D, Venance L, Lenkei Z. McFadden MH, et al. Transl Psychiatry. 2024 Aug 21;14(1):335. doi: 10.1038/s41398-024-03017-4. Transl Psychiatry. 2024. PMID: 39168993 Free PMC article. - Regulation of synaptic activity by snapin-mediated endolysosomal transport and sorting.
Di Giovanni J, Sheng ZH. Di Giovanni J, et al. EMBO J. 2015 Aug 4;34(15):2059-77. doi: 10.15252/embj.201591125. Epub 2015 Jun 24. EMBO J. 2015. PMID: 26108535 Free PMC article. - Dynamic Partitioning of Synaptic Vesicle Pools by the SNARE-Binding Protein Tomosyn.
Cazares VA, Njus MM, Manly A, Saldate JJ, Subramani A, Ben-Simon Y, Sutton MA, Ashery U, Stuenkel EL. Cazares VA, et al. J Neurosci. 2016 Nov 2;36(44):11208-11222. doi: 10.1523/JNEUROSCI.1297-16.2016. J Neurosci. 2016. PMID: 27807164 Free PMC article. - Synuclein Regulates Synaptic Vesicle Clustering and Docking at a Vertebrate Synapse.
Fouke KE, Wegman ME, Weber SA, Brady EB, Román-Vendrell C, Morgan JR. Fouke KE, et al. Front Cell Dev Biol. 2021 Nov 26;9:774650. doi: 10.3389/fcell.2021.774650. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34901020 Free PMC article. - Synapsins contribute to the dynamic spatial organization of synaptic vesicles in an activity-dependent manner.
Fornasiero EF, Raimondi A, Guarnieri FC, Orlando M, Fesce R, Benfenati F, Valtorta F. Fornasiero EF, et al. J Neurosci. 2012 Aug 29;32(35):12214-27. doi: 10.1523/JNEUROSCI.1554-12.2012. J Neurosci. 2012. PMID: 22933803 Free PMC article.
References
- Abbe E. (1873). Beiträge zur Theorie des Mikroskops und der mikroskopischen Wahrnehmung. Arch. Mikros. Anat. 9, 55
- Angleson J. K., Betz W. J. (2001). Intraterminal Ca(2+) and spontaneous transmitter release at the frog neuromuscular junction. J. Neurophysiol. 85, 287–294 - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous