Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors - PubMed (original) (raw)
. 2011 Mar 23;3(75):75ra26.
doi: 10.1126/scitranslmed.3002003.
Belinda A Waltman, Dora Dias-Santagata, Subba Digumarthy, Alexa B Turke, Panos Fidias, Kristin Bergethon, Alice T Shaw, Scott Gettinger, Arjola K Cosper, Sara Akhavanfard, Rebecca S Heist, Jennifer Temel, James G Christensen, John C Wain, Thomas J Lynch, Kathy Vernovsky, Eugene J Mark, Michael Lanuti, A John Iafrate, Mari Mino-Kenudson, Jeffrey A Engelman
Affiliations
- PMID: 21430269
- PMCID: PMC3132801
- DOI: 10.1126/scitranslmed.3002003
Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors
Lecia V Sequist et al. Sci Transl Med. 2011.
Abstract
Lung cancers harboring mutations in the epidermal growth factor receptor (EGFR) respond to EGFR tyrosine kinase inhibitors, but drug resistance invariably emerges. To elucidate mechanisms of acquired drug resistance, we performed systematic genetic and histological analyses of tumor biopsies from 37 patients with drug-resistant non-small cell lung cancers (NSCLCs) carrying EGFR mutations. All drug-resistant tumors retained their original activating EGFR mutations, and some acquired known mechanisms of resistance including the EGFR T790M mutation or MET gene amplification. Some resistant cancers showed unexpected genetic changes including EGFR amplification and mutations in the PIK3CA gene, whereas others underwent a pronounced epithelial-to-mesenchymal transition. Surprisingly, five resistant tumors (14%) transformed from NSCLC into small cell lung cancer (SCLC) and were sensitive to standard SCLC treatments. In three patients, serial biopsies revealed that genetic mechanisms of resistance were lost in the absence of the continued selective pressure of EGFR inhibitor treatment, and such cancers were sensitive to a second round of treatment with EGFR inhibitors. Collectively, these results deepen our understanding of resistance to EGFR inhibitors and underscore the importance of repeatedly assessing cancers throughout the course of the disease.
Figures
Fig. 1
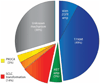
The frequency of observed drug resistance mechanisms. The pie chart depicts the prevalence of observed mechanisms of resistance to EGFR TKIs in 37 patients with NSCLC biopsied at the time that resistance was acquired. Pre- and posttreatment specimens were compared and only acquired mechanisms of resistance are depicted. The blue wedge represents resistant cancers that developed the EGFR T790M resistance mutation including a subset that developed concomitant EGFR amplification. The green wedge represents cancers that developed MET amplification, and the red wedge represents cancers that underwent transformation to SCLC. The yellow wedge represents cancers that developed PIK3CA mutations, and the orange wedge represents one patient who had both SCLC transformation and acquisition of a PIK3CA mutation.
Fig. 2
Acquired genetic amplifications in drug-resistant lung tumors. Amplification of MET and EGFR genes was observed in biopsies of tumors from patients who had acquired resistance to EGFR TKIs. Shown are FISH analyses that detect the MET gene (green), EGFR gene (red), and the control CEP7 gene (aqua). (A) The pretreatment specimen from patient 19 (left panel) shows a normal MET copy number but significant EGFR amplification; the drug-resistant posttreatment specimen (right panel) from the same patient exhibits acquired MET amplification but normal EGFR copy number. (B) Patient 13 demonstrated an acquired EGFR amplification in the drug-resistant posttreatment specimen (right panel) compared to the pretreatment specimen (left panel).
Fig. 3
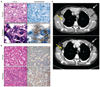
Drug resistance and transformation of NSCLC to SCLC. The SCLC histological phenotype was observed in five (14%) NSCLC patients who had acquired resistance. Two examples are shown. (A) Patient 23 had an exon 19 deletion in EGFR. (B) Patient 22 carried the L858R mutation in EGFR. The presence of the original activating mutation was confirmed in both pretreatment (pre-Rx) specimens (upper panels) and drug-resistant specimens (lower panels). Hematoxylin and eosin (H&E) (left) and synaptophysin (right) staining are shown for each case. Notably, the pretreatment biopsies (A and B, upper panels) demonstrate adenocarcinoma consisting of cellular growths of atypical glands with (A) or without (B) a cribriform pattern that are negative for synaptophysin. The post-resistance biopsies (A and B, lower panels) demonstrate a SCLC phenotype consisting of nests of small cells with a high nuclear-to-cytoplasmic ratio with (A) or without (B) the classic SCLC-associated finding of “crush artifact,” with positive immunohistochemical staining for synaptophysin. (C) Computed tomography scans of a representative patient (patient 25) with SCLC in the acquired resistance specimen before (above) and after (below) chemotherapy with cisplatin and etoposide (the standard regimen for treating SCLC). Yellow arrows denote right third rib metastases, and white arrows denote left axillary adenopathy.
Fig. 4
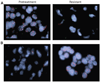
EMT and acquired resistance. (A) H1975 cells were cultured in the presence of the irreversible EGFR inhibitor PF00299804 until drug resistance developed, as demonstrated by Syto60 viability assays. (B) Images of the parental and drug-resistant H1975 cells by bright-field microscopy demonstrate that the resistant cells have developed a spindle-like morphology. (C) Parental and resistant H1975 cells were lysed and probed with antibodies against E-cadherin, vimentin, and actin, revealing loss of E-cadherin expression and gain of vi-mentin expression among drug-resistant H1975 cells. For comparison, HCC827 cells and the derived HCC827 GR6 cell line (HCC827 cells that acquired resistance to gefitinib via MET amplification), which do not undergo an EMT, are shown. (D) Example of a case (patient 28) whose drug-resistant tumor shows evidence of an EMT (top, pretreatment specimens; bottom, drug-resistant posttreatment specimens). Left, H&E staining; middle, staining for vimentin; right, staining for E-cadherin. Notably, the pretreatment cancer had an adenocarcino-ma histology (panel 1), does not stain for vimentin (panel 2), and shows preserved membranous staining with E-cadherin (panel 3). The vimentin-positive areas in panel 2 include alveolar macrophages (red circles), inflammatory and stromal cells in fibro-vascular cores (black arrows), but not tumor cells lining papillary structures (yellow arrows). The drug-resistant posttreatment specimen has sarcomatoid histology (panel 4), is positive for vimentin (panel 5), and is negative for E-cadherin (panel 6), consistent with an EMT.
Fig. 5
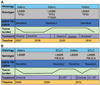
Longitudinal evaluation of patients treated repeatedly with erlotinib. The color-coded boxes to the left of each panel describe the data displayed across the timeline. The tumor burden depicted is not quantitative but represents tumor growth and shrinkage. (A) Patient 12 with a lung adenocar-cinoma carrying the L858R EGFR mutation and a mutation in the tumor suppressor p53 had a modest response to first-line chemotherapy. The patient then achieved a more robust and durable response to second-line erlotinib, with near-complete resolution of her lung nodules. After 8 months on an EGFR TKI, she developed resistance with growing bilateral pulmonary nodules. A lung core biopsy revealed an acquired T790M mutation in EGFR. There was no response to chemotherapy and she subsequently developed bone and liver metastases. At that time (after not taking the EGFR inhibitor for 8 months), a second lung core biopsy revealed the L858R EGFR mutation, but no detectable T790M EGFR resistace mutation. The patient responded to erlotinib (in combination with an investigational agent that did not target T790M). (B) Patient 24 had an L858R _EGFR_-mutant adenocarcinoma that responded markedly to first-line erlotinib for 12 months with resolution of her pleural effusion and pulmonary nodules. After 1 year, there was progression of the largest nodule. Core biopsy of this lesion revealed histological transformation to SCLC that harbored the EGFR L858R mutation and acquired a PIK3CA mutation. She was treated with radiation and chemotherapy. After a 6-month break from all treatment, her pleural effusion reaccumulated and small bilateral pulmonary nodules reappeared. Pleural effusion analysis revealed adenocarcinoma with the L858R EGFR mutation only (no PIK3CA mutation). Her disease responded to a second-line course of erlotinib. After 6 months, bony metastases and an adrenal lesion developed. Assessment of a growing bone metastasis revealed SCLC with both the L858R EGFR and PIK3CA mutations.
Comment in
- Resisting targeted therapy: fifty ways to leave your EGFR.
Workman P, Clarke PA. Workman P, et al. Cancer Cell. 2011 Apr 12;19(4):437-40. doi: 10.1016/j.ccr.2011.03.020. Cancer Cell. 2011. PMID: 21481786 - Targeted therapies: evolve and ... surrender!
Villanueva MT. Villanueva MT. Nat Rev Clin Oncol. 2011 Jun;8(6):315. doi: 10.1038/nrclinonc.2011.60. Epub 2011 May 3. Nat Rev Clin Oncol. 2011. PMID: 21544054 No abstract available.
Similar articles
- Acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancers dependent on the epidermal growth factor receptor pathway.
Nguyen KS, Kobayashi S, Costa DB. Nguyen KS, et al. Clin Lung Cancer. 2009 Jul;10(4):281-9. doi: 10.3816/CLC.2009.n.039. Clin Lung Cancer. 2009. PMID: 19632948 Free PMC article. Review. - Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers.
Yu HA, Arcila ME, Rekhtman N, Sima CS, Zakowski MF, Pao W, Kris MG, Miller VA, Ladanyi M, Riely GJ. Yu HA, et al. Clin Cancer Res. 2013 Apr 15;19(8):2240-7. doi: 10.1158/1078-0432.CCR-12-2246. Epub 2013 Mar 7. Clin Cancer Res. 2013. PMID: 23470965 Free PMC article. - Clinicopathologic and molecular features of epidermal growth factor receptor T790M mutation and c-MET amplification in tyrosine kinase inhibitor-resistant Chinese non-small cell lung cancer.
Chen HJ, Mok TS, Chen ZH, Guo AL, Zhang XC, Su J, Wu YL. Chen HJ, et al. Pathol Oncol Res. 2009 Dec;15(4):651-8. doi: 10.1007/s12253-009-9167-8. Epub 2009 Apr 21. Pathol Oncol Res. 2009. PMID: 19381876 - Primary resistance to epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) in patients with non-small-cell lung cancer harboring TKI-sensitive EGFR mutations: an exploratory study.
Lee JK, Shin JY, Kim S, Lee S, Park C, Kim JY, Koh Y, Keam B, Min HS, Kim TM, Jeon YK, Kim DW, Chung DH, Heo DS, Lee SH, Kim JI. Lee JK, et al. Ann Oncol. 2013 Aug;24(8):2080-7. doi: 10.1093/annonc/mdt127. Epub 2013 Apr 4. Ann Oncol. 2013. PMID: 23559152 - Activating and resistance mutations of EGFR in non-small-cell lung cancer: role in clinical response to EGFR tyrosine kinase inhibitors.
Gazdar AF. Gazdar AF. Oncogene. 2009 Aug;28 Suppl 1(Suppl 1):S24-31. doi: 10.1038/onc.2009.198. Oncogene. 2009. PMID: 19680293 Free PMC article. Review.
Cited by
- RBM15 facilitates osimertinib resistance of lung adenocarcinoma through m6A-dependent epigenetic silencing of SPOCK1.
Li H, Li Y, Zheng X, Chen F, Zhang S, Xu S, Mu Y, Shen W, Tong J, Chen H, Hu Z, Zhang J, Qiu K, Chen W, Cheng X, Xu G. Li H, et al. Oncogene. 2024 Nov 11. doi: 10.1038/s41388-024-03220-z. Online ahead of print. Oncogene. 2024. PMID: 39528815 - Histological transformation in lung adenocarcinoma: Insights of mechanisms and therapeutic windows.
Tan N, Li Y, Ying J, Chen W. Tan N, et al. J Transl Int Med. 2024 Nov 6;12(5):452-465. doi: 10.1515/jtim-2024-0019. eCollection 2024 Nov. J Transl Int Med. 2024. PMID: 39513032 Free PMC article. - Detailed characterization of combination treatment with MET inhibitor plus EGFR inhibitor in _EGFR_-mutant and _MET_-amplified non-small cell lung cancer.
Lee Y, Park SY, Lee GK, Lim HJ, Choi YR, Kim J, Han JY. Lee Y, et al. Transl Lung Cancer Res. 2024 Oct 31;13(10):2511-2523. doi: 10.21037/tlcr-24-273. Epub 2024 Oct 11. Transl Lung Cancer Res. 2024. PMID: 39507027 Free PMC article. - EGFR mutations in patients with lung adenocarcinoma and malignant pleural effusion: a propensity score-matched analysis of a single-center database.
Yang Q, Wang Z, Fu Q, Hu X, Chen L, Chen W, Lv L, Liu Z, Men W, Li D, Li W. Yang Q, et al. Transl Lung Cancer Res. 2024 Sep 30;13(9):2435-2447. doi: 10.21037/tlcr-24-757. Epub 2024 Sep 27. Transl Lung Cancer Res. 2024. PMID: 39430340 Free PMC article. - Clinical Management in NSCLC Patients With EGFR Mutation After Osimertinib Progression With Unknown Resistance Mechanisms.
Liao X, He T, Wan X, Liu P, Li J, He Y, Wang Y. Liao X, et al. Clin Respir J. 2024 Oct;18(10):e70025. doi: 10.1111/crj.70025. Clin Respir J. 2024. PMID: 39406371 Free PMC article.
References
- Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, Louis DN, Christiani DC, Settleman J, Haber DA. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 2004;350:2129–2139. - PubMed
- Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, Naoki K, Sasaki H, Fujii Y, Eck MJ, Sellers WR, Johnson BE, Meyerson M. EGFR mutations in lung cancer: Correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. - PubMed
- Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, Singh B, Heelan R, Rusch V, Fulton L, Mardis E, Kupfer D, Wilson R, Kris M, Varmus H. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc. Natl. Acad. Sci. U.S.A. 2004;101:13306–13311. - PMC - PubMed
- Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, Nishiwaki Y, Ohe Y, Yang JJ, Chewaskulyong B, Jiang H, Duffield EL, Watkins CL, Armour AA, Fukuoka M. Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. N. Engl. J. Med. 2009;361:947–957. - PubMed
- Rosell R, Moran T, Queralt C, Porta R, Cardenal F, Camps C, Majem M, Lopez-Vivanco G, Isla D, Provencio M, Insa A, Massuti B, Gonzalez-Larriba JL, Paz-Ares L, Bover I, Garcia-Campelo R, Moreno MA, Catot S, Rolfo C, Reguart N, Palmero R, Sánchez JM, Bastus R, Mayo C, Bertran-Alamillo J, Molina MA, Sanchez JJ, Taron M. Spanish Lung Cancer Group, Screening for epidermal growth factor receptor mutations in lung cancer. N. Engl. J. Med. 2009;361:958–967. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P20 CA090578/CA/NCI NIH HHS/United States
- P50CA090578/CA/NCI NIH HHS/United States
- R01 CA137008-01/CA/NCI NIH HHS/United States
- U01 CA141457-01/CA/NCI NIH HHS/United States
- K08 CA120060/CA/NCI NIH HHS/United States
- K08 GRANT CA120060-01/CA/NCI NIH HHS/United States
- K08 CA120060-01/CA/NCI NIH HHS/United States
- P50 CA127003-01/CA/NCI NIH HHS/United States
- P50 CA127003/CA/NCI NIH HHS/United States
- R01CA140594/CA/NCI NIH HHS/United States
- R01 CA137008/CA/NCI NIH HHS/United States
- U01 CA141457/CA/NCI NIH HHS/United States
- R21 CA156000-01/CA/NCI NIH HHS/United States
- R01 CA140594-01/CA/NCI NIH HHS/United States
- 1U01CA141457-01/CA/NCI NIH HHS/United States
- R01 CA140594/CA/NCI NIH HHS/United States
- P20 CA090578-02/CA/NCI NIH HHS/United States
- R01CA137008-01/CA/NCI NIH HHS/United States
- R21 CA156000/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous