FAS and NF-κB signalling modulate dependence of lung cancers on mutant EGFR - PubMed (original) (raw)
. 2011 Mar 24;471(7339):523-6.
doi: 10.1038/nature09870.
Haley Hieronymus, Joel Parker, Kenneth Chang, Miquel Taron, Rafael Rosell, Philicia Moonsamy, Kimberly Dahlman, Vincent A Miller, Carlota Costa, Gregory Hannon, Charles L Sawyers
Affiliations
- PMID: 21430781
- PMCID: PMC3541675
- DOI: 10.1038/nature09870
FAS and NF-κB signalling modulate dependence of lung cancers on mutant EGFR
Trever G Bivona et al. Nature. 2011.
Abstract
Human lung adenocarcinomas with activating mutations in EGFR (epidermal growth factor receptor) often respond to treatment with EGFR tyrosine kinase inhibitors (TKIs), but the magnitude of tumour regression is variable and transient. This heterogeneity in treatment response could result from genetic modifiers that regulate the degree to which tumour cells are dependent on mutant EGFR. Through a pooled RNA interference screen, we show that knockdown of FAS and several components of the NF-κB pathway specifically enhanced cell death induced by the EGFR TKI erlotinib in EGFR-mutant lung cancer cells. Activation of NF-κB through overexpression of c-FLIP or IKK (also known as CFLAR and IKBKB, respectively), or silencing of IκB (also known as NFKBIA), rescued EGFR-mutant lung cancer cells from EGFR TKI treatment. Genetic or pharmacologic inhibition of NF-κB enhanced erlotinib-induced apoptosis in erlotinib-sensitive and erlotinib-resistant EGFR-mutant lung cancer models. Increased expression of the NF-κB inhibitor IκB predicted for improved response and survival in EGFR-mutant lung cancer patients treated with EGFR TKI. These data identify NF-κB as a potential companion drug target, together with EGFR, in EGFR-mutant lung cancers and provide insight into the mechanisms by which tumour cells escape from oncogene dependence.
Figures
Figure 1. Mutant EGFR oncogene dependence requires downregulation of the FAS-NF-κB pathway
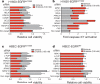
a, Viability (CellTiter-Glo assay) of H1650 cells treated with vehicle or 100 nM erlotinib upon introduction of either a non-target siRNA pool or gene-specific siRNA pools targeting the genes. Relative cell viability is fold change in viability in erlotinib relative to vehicle non-target siRNA control (viability decrease >25% = validated). n = 3; mean + s.e.m. b, Caspase 3/7 activation (Caspase-Glo assay) in indicated cell lines treated as in a. c, d, Viability (CellTiter-Glo assay) of indicated isogenic HBEC cells treated as in a. n = 3; mean + s.e.m.
Figure 2. Suppression of the FAS-NF-κB pathway enhances EGFR TKI response in EGFR-mutant cells and tumour models
a, Erlotinib dose response in H1650 cells expressing a non-target control shRNA or a FAS (shRNA2), RELA (shRNA1), or c-FLIP(shRNA1) shRNA (5 μM erlotinib IC50 in parental H1650 cells). Cell viability was assayed as in Fig. 1. n = 3; mean + s.e.m. b, Immunoblots showing expression of indicated signalling proteins in H1650 cells generated in a. Data represent three independent experiments. NT, non-target; PARPcl, PARP cleaved. (The decrease in c-FLIP protein by shRNA was less complete than the decrease in c-FLIP mRNA level by siRNA; Supplementary Fig. 1). c, Effects of stable knockdown of FAS (shRNA3) or RELA (shRNA2) on erlotinib sensitivity in H1650 xenograft tumours, compared to non-target shRNA control H1650 tumours. Established tumours (>200 mm3,, n = 10 per treatment group) were randomized and treated for 7 days with 12.5 mg erlotinib per kg per day, n = 10. Data expressed as mean + s.e.m. d, Immunoblots showing expression of indicated proteins in representative H1650 tumour xenografts from c analysed at treatment day 7.
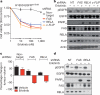
Figure 3. NF-κB activation through IκB downregulation confers EGFR TKI resistance in EGFR-mutant lung cancer models
a, Correlation of IκB expression with EGFR TKI sensitivity in EGFR-mutant lung cancer cells (IκB mRNA expression from Oncomine; sensitive <0.02 μM IC50: HCC827, H3255, HCC4006; resistant >1 μM IC50: H1650, H1975, H820). b, Immunoblots showing IκB and pRELA expression in lysates from HCC827 treated with non-targeting or IκB siRNA pools. c, Viability (CellTiter-Glo assay) of HCC827 cells treated with non-target siRNA pool or IκB siRNA pool and either vehicle or erlotinib (100 nM). RLU is relative luciferase units (n = 3; mean + s.e.m.). d, e, Effects of stable knockdown of IκB on erlotinib sensitivity in HCC827 tumour xenografts compared to non-target shRNA control HCC827 tumours. Tumours were established and treated as in Fig. 2c (n = 8 per treatment group, mean + s.e.m., 7-day treatment). f, Immunoblots showing expression of indicated proteins in representative HCC827 tumour xenografts analysed at treatment day 7.
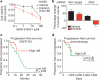
Figure 4. Rationale for combined NF-κB and EGFR inhibition in EGFR-mutant lung cancers
a, Dose response in H1650 cells treated with BMS-345541 (IKK inhibitor) and additionally either vehicle or erlotinib (100 nM). Viability was measured as in Fig. 1 (n = 3, mean + s.e.m.). b, Effects of stable knockdown of IKKβ on erlotinib sensitivity in H1650 tumour xenografts compared to non-target shRNA control H1650 tumours. Established tumours (>200 mm3, n = 10 per treatment group) were randomized and treated for 7 days with 12.5 mg erlotinib per kg per day or vehicle. Data are expressed as in Fig. 2c (+ s.e.m.). c, d, Effects of IκkB expression on progression free survival in patients with EGFR-mutant lung cancers (c) treated with single agent EGFR TKI (n = 52) or (d) chemotherapy and surgery (n = 43). Clinical characteristics and responses were defined previously. Median progression-free survival and overall survival for the entire EGFR TKI-treated cohort were 20 months (95% confidence interval, 13–26.9) and 33 months (95% confidence interval, 22.2–43.8), respectively.
Comment in
- Resisting targeted therapy: fifty ways to leave your EGFR.
Workman P, Clarke PA. Workman P, et al. Cancer Cell. 2011 Apr 12;19(4):437-40. doi: 10.1016/j.ccr.2011.03.020. Cancer Cell. 2011. PMID: 21481786
Similar articles
- NF-κB-driven improvement of EHD1 contributes to erlotinib resistance in EGFR-mutant lung cancers.
Wang X, Yin H, Zhang H, Hu J, Lu H, Li C, Cao M, Yan S, Cai L. Wang X, et al. Cell Death Dis. 2018 Apr 1;9(4):418. doi: 10.1038/s41419-018-0447-7. Cell Death Dis. 2018. PMID: 29549343 Free PMC article. - Progesterone receptor membrane component 1 leads to erlotinib resistance, initiating crosstalk of Wnt/β-catenin and NF-κB pathways, in lung adenocarcinoma cells.
Lin Y, Higashisaka K, Shintani T, Maki A, Hanamuro S, Haga Y, Maeda S, Tsujino H, Nagano K, Fujio Y, Tsutsumi Y. Lin Y, et al. Sci Rep. 2020 Mar 16;10(1):4748. doi: 10.1038/s41598-020-61727-3. Sci Rep. 2020. PMID: 32179851 Free PMC article. - Suppressed expression of Cbl-b by NF-κB mediates icotinib resistance in EGFR-mutant non-small-cell lung cancer.
Zhang T, Zheng C, Hou K, Wang J, Zhang Y, Fan Y, Zhao H, Qu X, Liu Y, Kang J, Che X, Hu X. Zhang T, et al. Cell Biol Int. 2019 Feb;43(2):98-107. doi: 10.1002/cbin.11026. Cell Biol Int. 2019. PMID: 29972257 - EGFR and NF-κB: partners in cancer.
Shostak K, Chariot A. Shostak K, et al. Trends Mol Med. 2015 Jun;21(6):385-93. doi: 10.1016/j.molmed.2015.04.001. Epub 2015 May 13. Trends Mol Med. 2015. PMID: 25979753 Review. - Fighting cancer drug resistance: Opportunities and challenges for mutation-specific EGFR inhibitors.
Juchum M, Günther M, Laufer SA. Juchum M, et al. Drug Resist Updat. 2015 May;20:12-28. doi: 10.1016/j.drup.2015.05.002. Epub 2015 May 12. Drug Resist Updat. 2015. PMID: 26021435 Review.
Cited by
- Epidermal growth factor receptor tyrosine kinase inhibitor-resistant disease.
Ohashi K, Maruvka YE, Michor F, Pao W. Ohashi K, et al. J Clin Oncol. 2013 Mar 10;31(8):1070-80. doi: 10.1200/JCO.2012.43.3912. Epub 2013 Feb 11. J Clin Oncol. 2013. PMID: 23401451 Free PMC article. Review. - Essential gene profiles in breast, pancreatic, and ovarian cancer cells.
Marcotte R, Brown KR, Suarez F, Sayad A, Karamboulas K, Krzyzanowski PM, Sircoulomb F, Medrano M, Fedyshyn Y, Koh JLY, van Dyk D, Fedyshyn B, Luhova M, Brito GC, Vizeacoumar FJ, Vizeacoumar FS, Datti A, Kasimer D, Buzina A, Mero P, Misquitta C, Normand J, Haider M, Ketela T, Wrana JL, Rottapel R, Neel BG, Moffat J. Marcotte R, et al. Cancer Discov. 2012 Feb;2(2):172-189. doi: 10.1158/2159-8290.CD-11-0224. Epub 2011 Dec 29. Cancer Discov. 2012. PMID: 22585861 Free PMC article. - Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer.
Zhang Z, Lee JC, Lin L, Olivas V, Au V, LaFramboise T, Abdel-Rahman M, Wang X, Levine AD, Rho JK, Choi YJ, Choi CM, Kim SW, Jang SJ, Park YS, Kim WS, Lee DH, Lee JS, Miller VA, Arcila M, Ladanyi M, Moonsamy P, Sawyers C, Boggon TJ, Ma PC, Costa C, Taron M, Rosell R, Halmos B, Bivona TG. Zhang Z, et al. Nat Genet. 2012 Jul 1;44(8):852-60. doi: 10.1038/ng.2330. Nat Genet. 2012. PMID: 22751098 Free PMC article. - RKIP-mediated chemo-immunosensitization of resistant cancer cells via disruption of the NF-κB/Snail/YY1/RKIP resistance-driver loop.
Bonavida B. Bonavida B. Crit Rev Oncog. 2014;19(6):431-45. doi: 10.1615/critrevoncog.2014011929. Crit Rev Oncog. 2014. PMID: 25597353 Free PMC article. Review. - Overcoming Tyrosine Kinase Inhibitor Resistance in Transformed Cell Harboring SEPT9-ABL1 Chimeric Fusion Protein.
Kawai H, Matsushita H, Suzuki R, Kitamura Y, Ogawa Y, Kawada H, Ando K. Kawai H, et al. Neoplasia. 2019 Aug;21(8):788-801. doi: 10.1016/j.neo.2019.06.001. Epub 2019 Jul 3. Neoplasia. 2019. PMID: 31276931 Free PMC article.
References
- Haber DA, et al. Molecular targeted therapy of lung cancer: EGFR mutations and response to EGFR inhibitors. Cold Spring Harb. Symp. Quant. Biol. 2005;70:419–426. - PubMed
- Miller VA, et al. Molecular characteristics of bronchioloalveolar carcinoma and adenocarcinoma, bronchioloalveolar carcinoma subtype, predict response to erlotinib. J. Clin. Oncol. 2008;26:1472–1478. - PubMed
- Weinstein IB, Joe A. Oncogene addiction. Cancer Res. 2008;68:3077–3080. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous