Interleukin-1β biosynthesis inhibition reduces acute seizures and drug resistant chronic epileptic activity in mice - PubMed (original) (raw)
Interleukin-1β biosynthesis inhibition reduces acute seizures and drug resistant chronic epileptic activity in mice
Mattia Maroso et al. Neurotherapeutics. 2011 Apr.
Abstract
Experimental evidence and clinical observations indicate that brain inflammation is an important factor in epilepsy. In particular, induction of interleukin-converting enzyme (ICE)/caspase-1 and activation of interleukin (IL)-1β/IL-1 receptor type 1 axis both occur in human epilepsy, and contribute to experimentally induced acute seizures. In this study, the anticonvulsant activity of VX-765 (a selective ICE/caspase-1 inhibitor) was examined in a mouse model of chronic epilepsy with spontaneous recurrent epileptic activity refractory to some common anticonvulsant drugs. Moreover, the effects of this drug were studied in one acute model of seizures in mice, previously shown to involve activation of ICE/caspase-1. Quantitative analysis of electroencephalogram activity was done in mice exposed to acute seizures or those developing chronic epileptic activity after status epilepticus to assess the anticonvulsant effects of systemic administration of VX-765. Histological and immunohistochemical analysis of brain tissue was carried out at the end of pharmacological experiments in epileptic mice to evaluate neuropathology, glia activation and IL-1β expression, and the effect of treatment. Repeated systemic administration of VX-765 significantly reduced chronic epileptic activity in mice in a dose-dependent fashion (12.5-200 mg/kg). This effect was observed at doses ≥ 50 mg/kg, and was reversible with discontinuation of the drug. Maximal drug effect was associated with inhibition of IL-1β synthesis in activated astrocytes. The same dose regimen of VX-765 also reduced acute seizures in mice and delayed their onset time. These results support a new target system for anticonvulsant pharmacological intervention to control epileptic activity that does not respond to some common anticonvulsant drugs.
Figures
FIG. 1
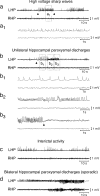
Representative electroencephalographic (EEG) tracings of acute seizures induced by intra-hippocampal kainate in vehicle- or VX-765-treated mice. Tracings in (a) and (c) depict baseline EEG activity recorded in the left hippocampi (LHP) and right hippocampi (RHP) before kainate injection in the LHP (7 ng in 0.5 μl). Tracings in (b) and (d) depict kainate-induced seizure activity (delimited by arrowheads) in mice pretreated with vehicle or 200 mg/kg i.p. VX-765, respectively. VX-765 reduced seizure number and duration as reported in Table 2.
FIG. 2
Representative electroencephalographic (EEG) tracings of chronic epileptic activity induced by intra-hippocampal kainate in mice. Chronic epileptic activity develops in mice 3 to 7 days after unilateral intra-hippocampal injection (left hippocampus [LHP]) of kainate (200 ng in 50 nl) causing SE. Tracings in (a) (boxed area is enlarged in a1) and (b) (boxed areas are enlarged in b1, b2, b3) depicting high-voltage sharp waves (HVSW) (1–4 mV; 3–8 Hz; average duration, 20 seconds) and unilateral hippocampal paroxysmal discharges (HPDs) that typically start (arrowhead in panel b) with large amplitude sharp waves (1–3 mV; 1–3 Hz; panel b1) followed by a train of spikes of increasing frequency (0.5–1.0 mV; 10–20 Hz, panels b2, b3), and terminating with a deflection in the EEG (arrowhead in panel b3). HVSW and HPDs were inhibited by VX-765 administration (see FIG. 4b–d). Panel (c) depicts interictal activity consisting of isolated spikes or spike trains (1–3 Hz; duration, <20 seconds); panel (d) shows bilateral hippocampal paroxysmal discharges (sporadic events in this model). The events in panels (c) and (d) were not included in the quantification of chronic epileptic activity. RHP = right hippocampus.
FIG. 3
Schematic representation of the treatment protocols in chronic epileptic mice. Panel (a) depicts the treatment protocol used in 5 randomly chosen epileptic mice: after establishing the baseline of spontaneous epileptic activity, the mice were treated intraperitoneally with a single dose of 50 mg/kg phenytoin. Then they underwent a 3-day wash-out period before entering protocol (b) (200 mg/kg VX-765). Panel (b) depicts the treatment protocol of 21 epileptic mice with VX-765: 3 days of electroencephalographic (EEG) baseline recording was done, and on day 4, the vehicle of VX-765 was injected intraperitoneally twice (at 9:00
AM
and 4:00
PM
), and on the following day, the epileptic mice received VX-765 twice a day (at 9:00
AM
and 4:00
PM
) for 4 consecutive days, and the EEG recording was done for 2 h after each drug administration. The last drug administration was followed by a 3-day wash-out period, and each day EEG was recorded (at 9:00
AM
to 11:00
AM)
to evaluate the time required by each epileptic mouse to recover its pre-injection baseline activity after drug withdrawal. Mice injected with VX-765 were killed for histological analysis (i.e., 5 mice at the time of maximal drug effect and 4 mice after drug wash-out). Panel (c) depicts the treatment protocol with dexamethasone: 6 epileptic mice at the end of treatment with 50 mg/kg VX-765, according to protocol (b), including the 3-day wash-out period were recorded by EEG continuously (between 9:00
AM
and 5:00
PM)
for 3 additional days (days 1–3) to monitor if the baseline epileptic activity had resumed and was stable. Then the vehicle was subcutaneously injected on day 4 (at 9:00
AM
and 4:00
PM
); on day 5, the baseline was again recorded (between 9:00
AM
and 11:00
AM)
, followed by a subcutaneous injection of dexamethasone in alcohol formulation (3 mg/kg). An EEG recording was continued for an additional 6 h (i.e., until 5:00
PM
). This procedure was repeated for 3 consecutive days. A 3-day wash-out period followed the last dexamethasone dose. d = day; DEX = dexamethasone.
FIG. 4
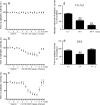
Panels (a–c): Anticonvulsant effects of VX-765 and dexamethasone in chronic epileptic mice. Electroencephalographic (EEG) activity was recorded and quantified for 3 days (days 1–3) in C57BL/6 epileptic mice, 6 weeks after kainate-induced status epilepticus (200 ng in 50 nl). Mice (n = 21) with a stable baseline of spontaneous epileptic activity (days 1–3; “see Table 1 for row values”) were injected daily intraperitoneally with 12.5 (n = 6), 50 (n = 6), or 200 mg/kg VX-765 (n = 9) (twice a day at 9:00
am
and at 4:00
pm
) from day 4 to day 7 (“see Methods for details”) (panels a–c). EEG activity was measured continuously after each drug injection for 2 h, then discontinued until the subsequent injection. In each experimental group, VX-765 treatment was withdrawn at day 7 (after the 4:00
pm
injection), and EEG recordings were made for the subsequent 3 days (days 8–10) to monitor when pre-injection baseline was resumed after drug wash-out. Panel (d) depicts the maximal reduction in epileptic activity, as assessed for each dose of VX-765 at day 7 (after the 4:00
pm
injection). Panel (e) shows data from a subgroup of epileptic mice (n = 6) treated with dexamethasone (3 mg/kg s.c.) for 3 days (“see Methods and FIG. 3c”). Data from day 1 of treatment only are shown because the same effect was observed in the subsequent 2 days. Data in panels (a–e) are presented as mean ± standard error of the mean of epileptic activity, expressed as a percentage of the corresponding pre-injection baseline (baseline raw data are shown in Table 1). *p < 0.05; **p < 0.01 versus baseline by repeated measures analysis of variance followed by Dunnett’s test. DEX = dexamethasone.
FIG. 5
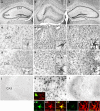
Histopathology, interleukin (IL)-1β expression, and glia activation in the hippocampus of epileptic mice, and effect of VX-765. Nissl-stained sections of the hippocampi of representative (a) vehicle-injected and (b, c) epileptic mice. (b) The hippocampus injected with kainate shows histopathology reminescent of hippocampal sclerosis, involving cell loss in pyramidal cell layers and degeneration of hilar interneurons, and dispersion of granule cells. (c) The contralateral non-injected side has normal histology similar to (a) vehicle-injected mice. (d–l) These panels show representative micrographs of CA3 hippocampal sections depicting (d–f) GFAP-positive astrocytes, (g–i) CD-11b-positive microglia, and (j–l) IL-1β immunostaining in (d, g, and j) vehicle-injected mice, and (e, f, h, i, k, and l) epileptic mice. (e, h, and k) Show the hippocampus of an epileptic mouse at the end of 200 mg/kg VX-765 wash-out period (i.e., at day 10 in FIG. 4c); (f, i, and l) show the hippocampus of a representative mouse killed at the time of maximal anticonvulsant drug effect (i.e., at day 7 in FIG. 4c). (d–i, insets) show 4-fold magnification of glial cells to show their phenotypic activation in (e, f, h, and i) epileptic tissue vs (d, g) control tissue. (h, inset) shows a 2-fold magnification of perivascular CD68-positive macrophages. Co-localization panels (k1–l3) show IL-1β expression in activated astrocytes in the hippocampus of epileptic mice; note the inhibition of IL-1β expression in VX-765 treated mice (l1–l3). IL-1β signal did not co-localize with CD11b signal denoting lack of IL-1β localization in microglia (k, inset). Scale bar in (a–c) 250 μm; (d–l) 100 μm. CA1 = Cornus Ammoni’s 1; CA3 = Cornus Ammoni’s 1; h = hilus.
Similar articles
- Interleukin Converting Enzyme inhibition impairs kindling epileptogenesis in rats by blocking astrocytic IL-1beta production.
Ravizza T, Noé F, Zardoni D, Vaghi V, Sifringer M, Vezzani A. Ravizza T, et al. Neurobiol Dis. 2008 Sep;31(3):327-33. doi: 10.1016/j.nbd.2008.05.007. Epub 2008 May 29. Neurobiol Dis. 2008. PMID: 18632279 - IL-1β is induced in reactive astrocytes in the somatosensory cortex of rats with genetic absence epilepsy at the onset of spike-and-wave discharges, and contributes to their occurrence.
Akin D, Ravizza T, Maroso M, Carcak N, Eryigit T, Vanzulli I, Aker RG, Vezzani A, Onat FY. Akin D, et al. Neurobiol Dis. 2011 Dec;44(3):259-69. doi: 10.1016/j.nbd.2011.05.015. Epub 2011 May 30. Neurobiol Dis. 2011. PMID: 21645619 - [Saikosaponin a alleviates pentylenetetrazol-induced acute epileptic seizures in mouse models of depression by suppressing microglia activation-mediated inflammation].
Xiong Y, Liang X, Liang X, Li W, Qian Y, Xie W. Xiong Y, et al. Nan Fang Yi Ke Da Xue Xue Bao. 2024 Mar 20;44(3):515-522. doi: 10.12122/j.issn.1673-4254.2024.03.13. Nan Fang Yi Ke Da Xue Xue Bao. 2024. PMID: 38597443 Free PMC article. Chinese. - ICE/caspase 1 inhibitors and IL-1beta receptor antagonists as potential therapeutics in epilepsy.
Vezzani A, Balosso S, Maroso M, Zardoni D, Noé F, Ravizza T. Vezzani A, et al. Curr Opin Investig Drugs. 2010 Jan;11(1):43-50. Curr Opin Investig Drugs. 2010. PMID: 20047158 Review. - The Search for New Screening Models of Pharmacoresistant Epilepsy: Is Induction of Acute Seizures in Epileptic Rodents a Suitable Approach?
Löscher W. Löscher W. Neurochem Res. 2017 Jul;42(7):1926-1938. doi: 10.1007/s11064-016-2025-7. Epub 2016 Aug 8. Neurochem Res. 2017. PMID: 27502939 Review.
Cited by
- Neuroinflammatory mediators in acquired epilepsy: an update.
Chen Y, Nagib MM, Yasmen N, Sluter MN, Littlejohn TL, Yu Y, Jiang J. Chen Y, et al. Inflamm Res. 2023 Apr;72(4):683-701. doi: 10.1007/s00011-023-01700-8. Epub 2023 Feb 6. Inflamm Res. 2023. PMID: 36745211 Free PMC article. Review. - Candidate drug targets for prevention or modification of epilepsy.
Varvel NH, Jiang J, Dingledine R. Varvel NH, et al. Annu Rev Pharmacol Toxicol. 2015;55:229-47. doi: 10.1146/annurev-pharmtox-010814-124607. Epub 2014 Aug 25. Annu Rev Pharmacol Toxicol. 2015. PMID: 25196047 Free PMC article. Review. - Role of inflammatory mediators in the pathogenesis of epilepsy.
Shimada T, Takemiya T, Sugiura H, Yamagata K. Shimada T, et al. Mediators Inflamm. 2014;2014:901902. doi: 10.1155/2014/901902. Epub 2014 Aug 13. Mediators Inflamm. 2014. PMID: 25197169 Free PMC article. Review. - Does brain inflammation mediate pathological outcomes in epilepsy?
Wilcox KS, Vezzani A. Wilcox KS, et al. Adv Exp Med Biol. 2014;813:169-83. doi: 10.1007/978-94-017-8914-1_14. Adv Exp Med Biol. 2014. PMID: 25012376 Free PMC article. Review. - HIV-1 Viral Protein R Activates NLRP3 Inflammasome in Microglia: implications for HIV-1 Associated Neuroinflammation.
Mamik MK, Hui E, Branton WG, McKenzie BA, Chisholm J, Cohen EA, Power C. Mamik MK, et al. J Neuroimmune Pharmacol. 2017 Jun;12(2):233-248. doi: 10.1007/s11481-016-9708-3. Epub 2016 Oct 10. J Neuroimmune Pharmacol. 2017. PMID: 27726055
References
- Ravizza T, Balosso S, Aronica E, Vezzani A. Brain Inflammation and epilepsy. In: Rho JM, Sankar R, Strafstrom CE, editors. Epilepsy: mechanisms, models, and translational perpsectives. Boca Raton: CRC Press; 2010. pp. 45–59.
- Eriksson C, Dam AM, Lucassen PJ, Bol JG, Winblad B, Schultzberg M. Immunohistochemical localization of interleukin-1beta, interleukin-1 receptor antagonist and interleukin-1beta converting enzyme/caspase-1 in the rat brain after peripheral administration of kainic acid. Neuroscience. 1999;93:915–930. doi: 10.1016/S0306-4522(99)00178-5. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical