BDNF control of adult SVZ neurogenesis - PubMed (original) (raw)
Review
BDNF control of adult SVZ neurogenesis
Kevin G Bath et al. Dev Psychobiol. 2012 Sep.
Abstract
The sensory processing of odorants is a dynamic process that requires plasticity at multiple levels. In the olfactory bulb (OB), inhibitory interneurons undergo lifelong replacement through a process known as adult neurogenesis. These newly born cells are incorporated in a learning-dependent fashion, a process which has led some to suggest this as a primary mechanism through which the OB retains a high degree of plasticity throughout life. A continued focus of researchers in this field has been to understand the molecular mechanisms controlling adult subventricular zone (SVZ) neurogenesis and the innate functional role of these cells. Brain-derived neurotrophic factor (BDNF) has been identified as a strong candidate molecule regulating adult OB neurogenesis. We review what is known regarding the functional role of newly born cells, highlight the role of BDNF in this process, and describe preliminary findings from our lab implicating BDNF in the process of selecting of newly born cells for survival.
Copyright © 2011 Wiley Periodicals, Inc.
Figures
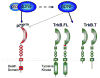
Figure 1. BDNF and its receptors
Brain derived neurotrophic factor (BDNF) is a member of the neurotrophin family of polypeptide growth factors. BDNF is synthesized from a 32 kDa precursor protein known as ProBDNF, which preferentially binds to the p75NTR neurotrophin receptor (Lee et al., 2001; Teng et al., 2005). ProBDNF binding to p75NTR and ensuing signaling has been linked with the induction of cell death and suppression of cell cycling in a variety of cellular populations. ProBDNF is cleaved to produce a mature 14 kDa form of BDNF which binds preferentially to the tropomysin related kinase receptor, TrkB (Chao, 2003). Mature BDNF signaling depends upon which of the 4 distinct isoforms of TrkB it binds to. There are two full-length forms of TrkB (TrkB.FL) which differ in a short amino acid insert on the extracellular domain, but both contain an active kinase domain with multiple phosphorylation sites. Activation of the full-length form of TrkB is thought to promote cellular differentiation, survival, neurite outgrowth, and synaptic plasticity (Reichardt, 2006). In addition, two truncated forms of TrkB have been identified, TrkB.T1 and TrkB.T2 which both lack the intracellular kinase domain (Stoilov et al., 2002; Reichardt, 2006). The truncated forms of TrkB are largely thought to function as endogenous dominant negatives (Eide et al., 1996; Haapasalo et al., 2001). However, recent work by Mattson and colleagues have identified a potential role for truncated TrkB in signaling via trans-activation of a G-protein coupled receptor (Cheng et al., 2007). In this model, truncated TrkB is though to function in the process of gliogenesis.
Figure 2. Developmental Expression of Neurotrophins
The expression of BDNF is dynamically regulated across development. In the OB of the mouse, BDNF levels are low during prenatal development and increase over the first weeks of life, peaking during adolescence and then declining across the remainder of life. Expression of p75NTR also changes dramatically over the lifespan. p75NTR is highly expressed prenatally and is then rapidly suppressed in most brain regions postnatally. The full-length form of the TrkB receptor appears be stably expressed across the lifespan, while TrkB.T1 expression appears to mirror that of BDNF, peaking during adolescence (unpublished data). The developmental peak in BDNF expression, occurs following the embryonic and early postnatal organization of the OB. This peak appears to correlate well with development of a proper RMS and the onset of adult-like neurogenesis.
Figure 3. Cell-specific deletion of BDNF impacts rates of OB neurogenesis
Histograms depicting the effects of selective genetic deletion of BDNF from A) Nestin-positive, B) Dlx5/6-positive, or C) CamKIIa-positive cells on rates of cell survival within the adult mouse OB. All groups were compared with Cre-positive mice that did not contain LoxP sites flanking the bdnf gene. Nestin-Cre BDNF LoxP groups were small (n=2) due to high postnatal mortality of this line. * p < 0.05.
Similar articles
- Brain-derived neurotrophic factor signaling does not stimulate subventricular zone neurogenesis in adult mice and rats.
Galvão RP, Garcia-Verdugo JM, Alvarez-Buylla A. Galvão RP, et al. J Neurosci. 2008 Dec 10;28(50):13368-83. doi: 10.1523/JNEUROSCI.2918-08.2008. J Neurosci. 2008. PMID: 19074010 Free PMC article. - Variant brain-derived neurotrophic factor (Val66Met) alters adult olfactory bulb neurogenesis and spontaneous olfactory discrimination.
Bath KG, Mandairon N, Jing D, Rajagopal R, Kapoor R, Chen ZY, Khan T, Proenca CC, Kraemer R, Cleland TA, Hempstead BL, Chao MV, Lee FS. Bath KG, et al. J Neurosci. 2008 Mar 5;28(10):2383-93. doi: 10.1523/JNEUROSCI.4387-07.2008. J Neurosci. 2008. PMID: 18322085 Free PMC article. - Brain-derived neurotrophic factor levels influence the balance of migration and differentiation of subventricular zone cells, but not guidance to the olfactory bulb.
Petridis AK, El Maarouf A. Petridis AK, et al. J Clin Neurosci. 2011 Feb;18(2):265-70. doi: 10.1016/j.jocn.2010.06.021. Epub 2010 Dec 21. J Clin Neurosci. 2011. PMID: 21177109 - Neurotrophic factor control of adult SVZ neurogenesis.
Bath KG, Lee FS. Bath KG, et al. Dev Neurobiol. 2010 Apr;70(5):339-49. doi: 10.1002/dneu.20781. Dev Neurobiol. 2010. PMID: 20186714 Free PMC article. Review. - Social Cues, Adult Neurogenesis, and Reproductive Behavior.
Peretto P, Paredes RG. Peretto P, et al. In: Mucignat-Caretta C, editor. Neurobiology of Chemical Communication. Boca Raton (FL): CRC Press/Taylor & Francis; 2014. Chapter 13. In: Mucignat-Caretta C, editor. Neurobiology of Chemical Communication. Boca Raton (FL): CRC Press/Taylor & Francis; 2014. Chapter 13. PMID: 24830028 Free Books & Documents. Review.
Cited by
- Potential effects of commonly applied drugs on neural stem cell proliferation and viability: A hypothesis-generating systematic review and meta-analysis.
Mortimer KRH, Vernon-Browne H, Zille M, Didwischus N, Boltze J. Mortimer KRH, et al. Front Mol Neurosci. 2022 Oct 5;15:975697. doi: 10.3389/fnmol.2022.975697. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36277493 Free PMC article. - Effects of smoking on cognition and BDNF levels in a male Chinese population: relationship with BDNF Val66Met polymorphism.
Xia H, Du X, Yin G, Zhang Y, Li X, Cai J, Huang X, Ning Y, Soares JC, Wu F, Zhang XY. Xia H, et al. Sci Rep. 2019 Jan 18;9(1):217. doi: 10.1038/s41598-018-36419-8. Sci Rep. 2019. PMID: 30659208 Free PMC article. - CRMP5 regulates generation and survival of newborn neurons in olfactory and hippocampal neurogenic areas of the adult mouse brain.
Veyrac A, Reibel S, Sacquet J, Mutin M, Camdessanche JP, Kolattukudy P, Honnorat J, Jourdan F. Veyrac A, et al. PLoS One. 2011;6(10):e23721. doi: 10.1371/journal.pone.0023721. Epub 2011 Oct 4. PLoS One. 2011. PMID: 21991301 Free PMC article. - Striatal astrocytes transdifferentiate into functional mature neurons following ischemic brain injury.
Duan CL, Liu CW, Shen SW, Yu Z, Mo JL, Chen XH, Sun FY. Duan CL, et al. Glia. 2015 Sep;63(9):1660-70. doi: 10.1002/glia.22837. Epub 2015 Jun 1. Glia. 2015. PMID: 26031629 Free PMC article. - Brain-derived neurotrophic factor promotes vasculature-associated migration of neuronal precursors toward the ischemic striatum.
Grade S, Weng YC, Snapyan M, Kriz J, Malva JO, Saghatelyan A. Grade S, et al. PLoS One. 2013;8(1):e55039. doi: 10.1371/journal.pone.0055039. Epub 2013 Jan 29. PLoS One. 2013. PMID: 23383048 Free PMC article.
References
- Altman J. Are new neurons formed in the brains of adult mammals? Science. 1962;135:1127–1128. - PubMed
- Altman J. Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol. 1969;137:433–457. - PubMed
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. JComp Neurol. 1965;124:319–335. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MH088814/MH/NIMH NIH HHS/United States
- RC1 MH088814-02/MH/NIMH NIH HHS/United States
- R00 MH090237/MH/NIMH NIH HHS/United States
- R01 NS052819/NS/NINDS NIH HHS/United States
- MH060478/MH/NIMH NIH HHS/United States
- K99 MH090237-02/MH/NIMH NIH HHS/United States
- MH090237/MH/NIMH NIH HHS/United States
- NS052819/NS/NINDS NIH HHS/United States
- R01 NS052819-05/NS/NINDS NIH HHS/United States
- RC1 MH088814/MH/NIMH NIH HHS/United States
- R25 MH060478/MH/NIMH NIH HHS/United States
- K99 MH090237/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous