Cilia in the CNS: the quiet organelle claims center stage - PubMed (original) (raw)
Review
Cilia in the CNS: the quiet organelle claims center stage
Angeliki Louvi et al. Neuron. 2011.
Abstract
The primary cilium is a cellular organelle that is almost ubiquitous in eukaryotes, yet its functions in vertebrates have been slow to emerge. The last fifteen years have been marked by accelerating insight into the biology of primary cilia, arising from the synergy of three major lines of research. These research programs describe a specialized mode of protein trafficking in cilia, reveal that genetic disruptions of primary cilia cause complex human disease syndromes, and establish that Sonic hedgehog (Shh) signal transduction requires the primary cilium. New lines of research have branched off to investigate the role of primary cilia in neuronal signaling, adult neurogenesis, and brain tumor formation. We review a fast expanding literature to determine what we now know about the primary cilium in the developing and adult CNS and what new directions should lead to further clarity.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
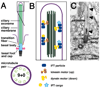
Figure 1. The structure of a primary cilium
(A) Major components are the ciliary axoneme, composed of microtubules (green), the ciliary membrane (purple) and the basal body (blue), which is a modified mother centriole. Modifications of the basal body include transition fibers (orange) that form a permeable barrier between the cilium and the rest of the cell, the basal foot and cap (pink) and striated rootlets (black horizontal lines), which provide mechanical support (Seeley and Nachury, 2010). A cross-section through the axoneme shows nine paired microtubules (the 9+0 configuration). (B) Macromolecules (sun shapes) important for ciliogenesis attach to IFT particles and travel along microtubules towards the ciliary tip using a kinesin motor. Turnover products (stars) are carried back to the ciliary base by IFT particles attached to a dynein motor. (C) Electronmicrograph of a primary cilium in an adult mouse brain. Visible features schematized in (A) include the axoneme, basal body, a transition fiber (tf), the basal foot and cap (bfc) and the daughter centriole (dc), which lies close to the basal body. Arrowheads indicate possible IFT particles travelling along the cilium. Scale bar in C is 0.5 microns.
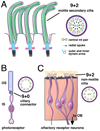
Figure 2. The structure of secondary and specialized sensory cilia
(A) Secondary cilia structurally resemble primary cilia, except that the axonemes of secondary cilia display a 9+2 microtubule configuration. The outer nine paired microtubules are attached to outer and inner dynein arms and connected to the central pair of tubules by radial spokes; this allows the secondary cilium self-generated motility. In the CNS, multiplesecondary cilia on ependymal cells lining the ventricles regulate the flow of cerebrospinal fluid (see text). (B) A ciliary segment joins the outer and inner segments (OS and IS) of the retinal photoreceptor and has the 9+0 configuration of a primary cilium. (C) Olfactory receptor neurons (ORNs) have cilia with a hybrid character. The cilia at the dendritic tips of each ORN display the 9+2 microtubule configuration, but lack the dynein machinery needed to generate motion (Arstila and Wersall, 1967; Jenkins et al., 2009). ORN cilia sample odorants in the mucus layer (yellow) at the surface of the olfactory epithelium (orange). ORN axons project to the olfactory bulb (OB).
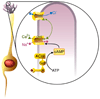
Figure 3. The cilium as a sensory transduction organelle
At left an ORN extends a dendrite ending in a cluster of cilia (purple). To the right, signal transduction in an ORN cilium. An odorant (red ball) binds to the olfactory receptor (R), coupled to the G-protein Golf. Activation of ACIII increases cAMP. cAMP opens CNG ion channels, causing an influx of Ca++ (green) and Na+ (red) ions which depolarizes the ORN. Raised Ca++ levels open Cl− (blue) channels, allowing an efflux of Cl−, further depolarizing the cell, and amplifying the odorant signal. The depolarized potential of the cilium spreads passively to the somatic membrane of the ORN where it activates Ca++, Na+ and K- channels, leading to the firing of an action potential.
Similar articles
- Hedgehog trafficking, cilia and brain functions.
Ruat M, Roudaut H, Ferent J, Traiffort E. Ruat M, et al. Differentiation. 2012 Feb;83(2):S97-104. doi: 10.1016/j.diff.2011.11.011. Epub 2011 Dec 9. Differentiation. 2012. PMID: 22169886 Review. - Hedgehog signaling and the primary cilium: implications for spatial and temporal constraints on signaling.
Ho EK, Stearns T. Ho EK, et al. Development. 2021 May 1;148(9):dev195552. doi: 10.1242/dev.195552. Epub 2021 Apr 29. Development. 2021. PMID: 33914866 Free PMC article. Review. - Mouse Kif7/Costal2 is a cilia-associated protein that regulates Sonic hedgehog signaling.
Liem KF Jr, He M, Ocbina PJ, Anderson KV. Liem KF Jr, et al. Proc Natl Acad Sci U S A. 2009 Aug 11;106(32):13377-82. doi: 10.1073/pnas.0906944106. Epub 2009 Jul 29. Proc Natl Acad Sci U S A. 2009. PMID: 19666503 Free PMC article. - Transient Primary Cilia Mediate Robust Hedgehog Pathway-Dependent Cell Cycle Control.
Ho EK, Tsai AE, Stearns T. Ho EK, et al. Curr Biol. 2020 Jul 20;30(14):2829-2835.e5. doi: 10.1016/j.cub.2020.05.004. Epub 2020 Jun 11. Curr Biol. 2020. PMID: 32531277 Free PMC article. - Primary cilium and sonic hedgehog signaling during neural tube patterning: role of GPCRs and second messengers.
Pal K, Mukhopadhyay S. Pal K, et al. Dev Neurobiol. 2015 Apr;75(4):337-48. doi: 10.1002/dneu.22193. Epub 2014 Jun 5. Dev Neurobiol. 2015. PMID: 24863049 Review.
Cited by
- Ofd1 controls dorso-ventral patterning and axoneme elongation during embryonic brain development.
D'Angelo A, De Angelis A, Avallone B, Piscopo I, Tammaro R, Studer M, Franco B. D'Angelo A, et al. PLoS One. 2012;7(12):e52937. doi: 10.1371/journal.pone.0052937. Epub 2012 Dec 27. PLoS One. 2012. PMID: 23300826 Free PMC article. - Arl13b in primary cilia regulates the migration and placement of interneurons in the developing cerebral cortex.
Higginbotham H, Eom TY, Mariani LE, Bachleda A, Hirt J, Gukassyan V, Cusack CL, Lai C, Caspary T, Anton ES. Higginbotham H, et al. Dev Cell. 2012 Nov 13;23(5):925-38. doi: 10.1016/j.devcel.2012.09.019. Dev Cell. 2012. PMID: 23153492 Free PMC article. - CCP1, a Tubulin Deglutamylase, Increases Survival of Rodent Spinal Cord Neurons following Glutamate-Induced Excitotoxicity.
Ramadan YH, Gu A, Ross N, McEwan SA, Barr MM, Firestein BL, O'Hagan R. Ramadan YH, et al. eNeuro. 2021 Apr 1;8(2):ENEURO.0431-20.2021. doi: 10.1523/ENEURO.0431-20.2021. Print 2021 Mar-Apr. eNeuro. 2021. PMID: 33688040 Free PMC article. - Somatostatin Serves a Modulatory Role in the Mouse Olfactory Bulb: Neuroanatomical and Behavioral Evidence.
Nocera S, Simon A, Fiquet O, Chen Y, Gascuel J, Datiche F, Schneider N, Epelbaum J, Viollet C. Nocera S, et al. Front Behav Neurosci. 2019 Apr 9;13:61. doi: 10.3389/fnbeh.2019.00061. eCollection 2019. Front Behav Neurosci. 2019. PMID: 31024270 Free PMC article. - Centrosome anchoring regulates progenitor properties and cortical formation.
Shao W, Yang J, He M, Yu XY, Lee CH, Yang Z, Joyner AL, Anderson KV, Zhang J, Tsou MB, Shi H, Shi SH. Shao W, et al. Nature. 2020 Apr;580(7801):106-112. doi: 10.1038/s41586-020-2139-6. Epub 2020 Mar 25. Nature. 2020. PMID: 32238932 Free PMC article.
References
- Albrecht-Buehler G. Phagokinetic tracks of 3T3 cells: parallels between the orientation of track segments and of cellular structures which contain actin or tubulin. Cell. 1977;12:333–339. - PubMed
- Angers S, Moon RT. Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol. 2009;10:468–477. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HD042330/HD/NICHD NIH HHS/United States
- R01 HD042330-09/HD/NICHD NIH HHS/United States
- R37 MH059962/MH/NIMH NIH HHS/United States
- R37 MH059962-11/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources