T cell responses during influenza infection: getting and keeping control - PubMed (original) (raw)
Review
T cell responses during influenza infection: getting and keeping control
Taeg S Kim et al. Trends Immunol. 2011 May.
Abstract
The 2009 influenza pandemic highlighted the threat that type A influenza poses to human health. Thus, there is an urgency to understand the pathobiology of influenza infection and the contribution of the host immune response to virus elimination and the development of lung injury. This review focuses on the T cell arm of the adaptive host immune response to influenza. We assess recent developments in the understanding of how primary influenza virus-specific T cell responses are induced by antigen-presenting cells, the interaction of activated effector T cells with antigen-bearing cells in the infected lungs. Also examined is the contribution of influenza-specific effector T cells to the development and control of lung injury and inflammation during infection.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures
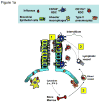
Figure 1. Migrant respiratory dendritic cell (RDC) antigen uptake, migration and T cell activation in response to influenza virus infection
(a) Influenza virus enters the respiratory tract and replicates (undergoes “productive” infection) in the respiratory epithelial cells lining the airways and, in more severe infections, the alveolar lining type I and II pneumocytes (1). The viral antigens generated are sampled by distinct subsets of resident RDCs that are located within or at the margins of the epithelial mucosal lining (i.e., CD103+ RDCs) or within the pulmonary interstitium (i.e., CD11bhi RDCs). Antigen acquisition by RDCs, along with virus-induced local inflammatory stimuli, triggers upregulation of CC-chemokine receptor 7 on the responding RDCs, which enables the chemokine-dependent migration of the activated RDC from the infected lung through the afferent lymphatics to the lung-draining lymph nodes (DLN) (2). IAV infection of epithelial cells along with virus-induced activation of lung resident innate immune cells orchestrates the recruitment of CD45+ innate immune/inflammatory cells from the circulation (and ultimately the bone marrow) to the site of infection (3). (b) Antigen-bearing migrant CD103+ and CD11bhi RDCs within the DLN (4) present processed IAV antigenic peptides to naive virus-specific T cells (5). Activated T cells undergo clonal expansion and differentiate into effectors capable of migrating through the efferent lymphatics and into the blood via the thoracic duct (6) and ultimately home to the infected lungs where they exert their anti-viral effector activity.
Figure 1. Migrant respiratory dendritic cell (RDC) antigen uptake, migration and T cell activation in response to influenza virus infection
(a) Influenza virus enters the respiratory tract and replicates (undergoes “productive” infection) in the respiratory epithelial cells lining the airways and, in more severe infections, the alveolar lining type I and II pneumocytes (1). The viral antigens generated are sampled by distinct subsets of resident RDCs that are located within or at the margins of the epithelial mucosal lining (i.e., CD103+ RDCs) or within the pulmonary interstitium (i.e., CD11bhi RDCs). Antigen acquisition by RDCs, along with virus-induced local inflammatory stimuli, triggers upregulation of CC-chemokine receptor 7 on the responding RDCs, which enables the chemokine-dependent migration of the activated RDC from the infected lung through the afferent lymphatics to the lung-draining lymph nodes (DLN) (2). IAV infection of epithelial cells along with virus-induced activation of lung resident innate immune cells orchestrates the recruitment of CD45+ innate immune/inflammatory cells from the circulation (and ultimately the bone marrow) to the site of infection (3). (b) Antigen-bearing migrant CD103+ and CD11bhi RDCs within the DLN (4) present processed IAV antigenic peptides to naive virus-specific T cells (5). Activated T cells undergo clonal expansion and differentiate into effectors capable of migrating through the efferent lymphatics and into the blood via the thoracic duct (6) and ultimately home to the infected lungs where they exert their anti-viral effector activity.
Figure 2. Interactions between antigen-presenting cells (APCs) and antiviral effector T cells (Te) in the influenza-infected lungs
CD45+ inflammatory/innate immune cells recruited to the IAV-infected lungs can participate in the host response to infection as mediators and regulators of pulmonary injury, through the production of pro-inflammatory and regulatory molecules (see Table 1), as well as APCs capable of enhancing Te function and proliferation through antigen-dependent APC-Te interactions. This antigen presenting activity appears to be mainly restricted to recruited inflammatory DCs and possibly monocytes/macrophages. APCs produce IL-15 and other molecules, which also facilitate Te cell recruitment to and survival in the infected lungs. The outcome of the encounter between the Te and the APC in the infected lung interstitium (e.g., production of pro-inflammatory cytokines by the Te cells) is controlled by TCR engagement, recognition of peptide/MHC complexes, and by co-stimulatory (and possibly co-inhibitory) receptor/ligand interactions between the Te and the APC within the infected lung interstitium and airways. The proinflammatory cytokines produced by Te cells contribute to lung inflammation while the regulatory cytokines, such as IL-10, released by Te cells help to restrain the excessive pulmonary inflammation and injury associated with more severe IAV infection. Recent evidence raises the possibility that the interaction of CD8+ Te with IAV-infected respiratory epithelium might result in the expression of only a subset of Te effector activities (e.g., cytolysis).
Similar articles
- The effector T cell response to influenza infection.
Hufford MM, Kim TS, Sun J, Braciale TJ. Hufford MM, et al. Curr Top Microbiol Immunol. 2015;386:423-55. doi: 10.1007/82_2014_397. Curr Top Microbiol Immunol. 2015. PMID: 25033753 Free PMC article. Review. - Impaired immune responses in the lungs of aged mice following influenza infection.
Toapanta FR, Ross TM. Toapanta FR, et al. Respir Res. 2009 Nov 18;10(1):112. doi: 10.1186/1465-9921-10-112. Respir Res. 2009. PMID: 19922665 Free PMC article. - Role of T cell immunity in recovery from influenza virus infection.
Sun J, Braciale TJ. Sun J, et al. Curr Opin Virol. 2013 Aug;3(4):425-9. doi: 10.1016/j.coviro.2013.05.001. Epub 2013 May 27. Curr Opin Virol. 2013. PMID: 23721865 Free PMC article. Review. - Differential requirement for CD80 and CD80/CD86-dependent costimulation in the lung immune response to an influenza virus infection.
Lumsden JM, Roberts JM, Harris NL, Peach RJ, Ronchese F. Lumsden JM, et al. J Immunol. 2000 Jan 1;164(1):79-85. doi: 10.4049/jimmunol.164.1.79. J Immunol. 2000. PMID: 10604996 - High ambient temperature dampens adaptive immune responses to influenza A virus infection.
Moriyama M, Ichinohe T. Moriyama M, et al. Proc Natl Acad Sci U S A. 2019 Feb 19;116(8):3118-3125. doi: 10.1073/pnas.1815029116. Epub 2019 Feb 4. Proc Natl Acad Sci U S A. 2019. PMID: 30718396 Free PMC article.
Cited by
- A specific and portable gene expression program underlies antigen archiving by lymphatic endothelial cells.
Sheridan RM, Doan TA, Lucas C, Forward TS, Uecker-Martin A, Morrison TE, Hesselberth JR, Tamburini BAJ. Sheridan RM, et al. bioRxiv [Preprint]. 2024 Apr 2:2024.04.01.587647. doi: 10.1101/2024.04.01.587647. bioRxiv. 2024. PMID: 38617225 Free PMC article. Preprint. - Immunization-induced antigen archiving enhances local memory CD8+ T cell responses following an unrelated viral infection.
Doan TA, Forward TS, Schafer JB, Lucas ED, Fleming I, Uecker-Martin A, Ayala E, Guthmiller JJ, Hesselberth JR, Morrison TE, Tamburini BAJ. Doan TA, et al. NPJ Vaccines. 2024 Mar 21;9(1):66. doi: 10.1038/s41541-024-00856-6. NPJ Vaccines. 2024. PMID: 38514656 Free PMC article. - Vaccine-induced antigen archiving enhances local memory CD8+ T cell responses following an unrelated viral infection.
Tamburini B, Doan T, Forward T, Lucas E, Fleming I, Uecker-Martin A, Hesselberth J, Morrison T. Tamburini B, et al. Res Sq [Preprint]. 2023 Sep 25:rs.3.rs-3307809. doi: 10.21203/rs.3.rs-3307809/v1. Res Sq. 2023. PMID: 37841845 Free PMC article. Updated. Preprint. - Antigen-specific and cross-reactive T cells in protection and disease.
Jiang N, Malone M, Chizari S. Jiang N, et al. Immunol Rev. 2023 Jul;316(1):120-135. doi: 10.1111/imr.13217. Epub 2023 May 20. Immunol Rev. 2023. PMID: 37209375 Free PMC article. Review. - Tissue-resident memory T cells and lung immunopathology.
Cheon IS, Son YM, Sun J. Cheon IS, et al. Immunol Rev. 2023 Jul;316(1):63-83. doi: 10.1111/imr.13201. Epub 2023 Apr 4. Immunol Rev. 2023. PMID: 37014096 Free PMC article. Review.
References
- Maines TR, et al. Pathogenesis of emerging avian influenza viruses in mammals and the host innate immune response. Immunol Rev. 2008;225:68–84. - PubMed
- Sanders CJ, et al. Respiratory epithelial cells in innate immunity to influenza virus infection. Cell Tissue Res 2010 - PubMed
- Holt PG, et al. Regulation of immunological homeostasis in the respiratory tract. Nat Rev Immunol. 2008;8:142–152. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 AI037293/AI/NIAID NIH HHS/United States
- R01 AI-37293/AI/NIAID NIH HHS/United States
- R01 HL-33391/HL/NHLBI NIH HHS/United States
- U-19 AI-83024/AI/NIAID NIH HHS/United States
- R37 AI015608/AI/NIAID NIH HHS/United States
- R01 HL033391/HL/NHLBI NIH HHS/United States
- R01 AI-15608/AI/NIAID NIH HHS/United States
- U19 AI083024/AI/NIAID NIH HHS/United States
- R01 AI037293-12/AI/NIAID NIH HHS/United States
- R01 HL033391-27/HL/NHLBI NIH HHS/United States
- R01 HL071875-04/HL/NHLBI NIH HHS/United States
- R37 AI015608-21/AI/NIAID NIH HHS/United States
- R01 AI015608-31/AI/NIAID NIH HHS/United States
- R01 HL071875/HL/NHLBI NIH HHS/United States
- U19 AI083024-01/AI/NIAID NIH HHS/United States
- R01 AI015608/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical