Immunotherapeutic effects of IL-7 during a chronic viral infection in mice - PubMed (original) (raw)
Immunotherapeutic effects of IL-7 during a chronic viral infection in mice
Som G Nanjappa et al. Blood. 2011.
Abstract
Viral persistence during chronic viral infections is associated with a progressive loss of T-cell effector function called functional exhaustion. There is therefore a need to develop immunotherapies to remediate the functional deficits of T cells during these infections. We investigated the immunotherapeutic effects of IL-7 during chronic lymphocytic choriomeningitis virus infection in mice. Our results showed that the effects of IL-7 on T cells depend on the viral load, timing, and duration of treatment during the course of the infection. We document that the effectiveness of IL-7 was constrained by high viral load early in the infection, but treatment for at least 3 weeks during declining viral titers mitigated the programmed contraction of CD8 T cells, markedly enhanced the number of high-quality polyfunctional virus-specific CD8 T cells with a nonexhausted phenotype, and accelerated viral control. Mechanistically, the enhancement of CD8 T-cell responses by IL-7 was associated with increased proliferation and induction of Bcl-2, but not with altered levels of PD-1 or Cbl-b. In summary, our results strongly suggest that IL-7 therapy is a potential strategy to bolster the quality and quantity of T-cell responses in patients with chronic viral infections.
Figures
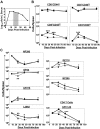
Figure 1
Effect of IL-7 treatment during early contraction phase on the CD8 T-cell response. Cohorts of LCMV-Clone 13–infected mice were treated daily with either IL-7 or PBS between days 8 and 15 PI, as depicted by the shaded area (A). At days 8 (before IL-7 therapy), 16 (1 day after completion of therapy), and 40 (25 days after cessation of IL-7 therapy) PI, the numbers of naive (CD44lo) and activated (CD44hi) CD8 or CD4 T cells (B) and LCMV epitope-specific, IFNγ-producing CD8 or CD4 T cells (C) in IL-7- (●) or PBS (▴)-treated mice were quantified by flow cytometry. Data for each time point were obtained from 4-5 mice per group. *P ≤ .05; **P ≤ .005.
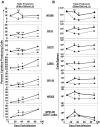
Figure 2
IL-7 therapy during the late contraction phase augments LCMV-specific T-cell responses. LCMV-Clone 13–infected mice were treated daily with either IL-7 or PBS between days 15 and 25 PI, as illustrated by the shaded area (A). At days 8, 26 (1 day after cessation of therapy), 45 (20 days after completion of IL-7 therapy), and 85 (60 days after IL-7 therapy) PI, CD8 and CD4 T-cell responses in the spleens of IL-7 (●)– or PBS (▴)–treated mice were quantified by flow cytometry. (B) The numbers of naive and activated CD8 or CD4 T cells. (C) LCMV epitope-specific CD8/CD4 T cells were quantified by intracellular staining for IFNγ. Data for each time point was obtained from 4-5 mice per group. *P ≤ .05; **P ≤ .005; ***P ≤ .001.
Figure 3
Extended duration of IL-7 therapy during clonal contraction enhances the LCMV-specific T-cell response. Cohorts of LCMV-Clone 13–infected mice were treated daily with either IL-7 or PBS between days 8 and 30 PI, as illustrated by the shaded area (A). At days 8, 31, 44, and 90 PI, LCMV-specific CD8 and CD4 T-cell responses were quantified by flow cytometry. (B) The kinetics of naive and activated CD8 or CD4 T cells in IL-7– and PBS–treated mice. (C) LCMV epitope-specific CD8 and CD4 T cells were quantified by staining for intracellular IFNγ. Data for each time point was obtained from an analysis of 4-5 IL-7 (●)– or PBS (▴)–treated mice and are representative of 2 experiments. *P ≤ .05; **P ≤ .005; ***P ≤ .001.
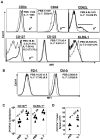
Figure 4
Extended duration of IL-7 treatment during the clonal contraction phase alters phenotypic attributes of LCMV-specific CD8 T cells. Mice were infected with LCMV-Clone 13 and treated with either IL-7 or PBS between days 8 and 30 PI, as described in Figure 3. At day 31 PI, splenocytes were stained with anti-CD8, anti-CD44, and Db/GP33 tetramers in combination with antibodies against KLRG-1, CD127, CD122, CD43, PD-1, and LAG-3 (A-C). Splenocytes were stained with anti-CD8, Db/GP33 tetramers, and antibodies against intracellular Bcl-2, Cbl-b, and Ki-67 molecules (D). Data were analyzed by flow cytometry, and the FACS plots are gated on tetramer-binding CD8 T cells. The numbers in panels A, C, and D are the mean fluorescence intensity (MFI) and/or percentages among tetramer-binding CD8 T cells. Data are from analysis of 4-5 IL-7–treated (black line) or PBS-treated (gray line) mice and are representative of 2 experiments. Stainings with anti–Cbl-b antibodies after incubation with the specific immunogenic Cbl-b peptide are shown as dotted lines. *P ≤ .05; **P ≤ .005; ***P ≤ .001.
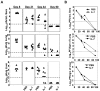
Figure 5
Extended duration of IL-7 treatment during the clonal contraction phase results in durable enhancement of the quality of LCMV-specific CD8 T cells. Mice were infected with LCMV-Clone 13 and treated with either IL-7 (●) or PBS (▴) between days 8 and 30 PI, as described in Figure 3. At days 8, 31, 44, and 90 PI, LCMV-specific CD8− and CD4− T-cell responses in the spleen were assessed by flow cytometry. LCMV epitope–specific, triple cytokine (IFNγ, TNFα, and IL-2)–producing cells were enumerated by intracellular staining (A-B). (A) The percentages of triple cytokine–producing cells of epitope-specific CD8 or CD4 T cells at different days PI. (B) The total numbers of triple cytokine–producing epitope-specific CD8 or CD4 cells. *P ≤ .05; **P ≤ .005; ***P ≤ .001.
Figure 6
Extended duration of IL-7 treatment during the clonal contraction phase alters the phenotypic attributes of LCMV-specific CD8 T cells. Mice were infected with LCMV-Clone 13 and treated with either IL-7 or PBS between days 8 and 30 PI, as described in Figure 3. At day 90 PI, splenocytes were stained with anti-CD8, anti-CD44, anti-CD122, anti-CD127, anti-CD62L, anti-KLRG-1, anti–Cbl-b, anti-PD-1, and Db/GP33 tetramers (A-C). (D) At day 44 PI (14 days after cessation of IL-7 therapy), splenocytes were incubated with anti-CD107a (anti–LAMP-1) and DbGP33 tetramer and stimulated with LCMV-specific cognate peptide for 1 hour at 37°C. After incubation, cells were washed and stained with anti-CD8 antibody. Data were analyzed by flow cytometry, and FACS plots were gated on tetramer-binding CD8 T cells. The numbers in panels A and B are the mean fluorescence intensity (MFI) and/or percentages of tetramer-binding CD8 T cells. Data are from an analysis of 4-5 IL-7–treated (black line) or PBS-treated (gray line) mice. Stainings with anti–Cbl-b antibodies preincubated with the specific immunogenic Cbl-b peptide are shown as dotted lines. *P ≤ .05; **P ≤ .005; ***P ≤ .001.
Figure 7
Extended duration of IL-7 treatment after the clonal expansion phase promotes accelerated viral clearance. Mice were infected with LCMV-Clone 13 and treated with either IL-7 or PBS as described in Figure 3. At days 8, 31, 44, and 90 PI, the virus titers in tissues of IL-7–treated or PBS-treated mice were quantified by plaque assay. Panel A shows virus titers in serum, lungs, and liver at different days PI in PBS- or IL-7–treated mice, and panel B illustrates the overall kinetics of viral clearance.
Similar articles
- PD-L1 Checkpoint Inhibition Narrows the Antigen-Specific T Cell Receptor Repertoire in Chronic Lymphocytic Choriomeningitis Virus Infection.
Klein S, Ghersi D, Manns MP, Prinz I, Cornberg M, Kraft ARM. Klein S, et al. J Virol. 2020 Aug 31;94(18):e00795-20. doi: 10.1128/JVI.00795-20. Print 2020 Aug 31. J Virol. 2020. PMID: 32641478 Free PMC article. - A vital role for interleukin-21 in the control of a chronic viral infection.
Yi JS, Du M, Zajac AJ. Yi JS, et al. Science. 2009 Jun 19;324(5934):1572-6. doi: 10.1126/science.1175194. Epub 2009 May 14. Science. 2009. PMID: 19443735 Free PMC article. - Effects of IL-7 on memory CD8 T cell homeostasis are influenced by the timing of therapy in mice.
Nanjappa SG, Walent JH, Morre M, Suresh M. Nanjappa SG, et al. J Clin Invest. 2008 Mar;118(3):1027-39. doi: 10.1172/JCI32020. J Clin Invest. 2008. PMID: 18246202 Free PMC article. - STAT5 is critical to maintain effector CD8+ T cell responses.
Tripathi P, Kurtulus S, Wojciechowski S, Sholl A, Hoebe K, Morris SC, Finkelman FD, Grimes HL, Hildeman DA. Tripathi P, et al. J Immunol. 2010 Aug 15;185(4):2116-24. doi: 10.4049/jimmunol.1000842. Epub 2010 Jul 19. J Immunol. 2010. PMID: 20644163 Free PMC article. - CD8 T cell dysfunction during chronic viral infection.
Shin H, Wherry EJ. Shin H, et al. Curr Opin Immunol. 2007 Aug;19(4):408-15. doi: 10.1016/j.coi.2007.06.004. Epub 2007 Jul 25. Curr Opin Immunol. 2007. PMID: 17656078 Review.
Cited by
- Safety and long-term improvement of mesenchymal stromal cell infusion in critically COVID-19 patients: a randomized clinical trial.
Rebelatto CLK, Senegaglia AC, Franck CL, Daga DR, Shigunov P, Stimamiglio MA, Marsaro DB, Schaidt B, Micosky A, de Azambuja AP, Leitão CA, Petterle RR, Jamur VR, Vaz IM, Mallmann AP, Carraro Junior H, Ditzel E, Brofman PRS, Correa A. Rebelatto CLK, et al. Stem Cell Res Ther. 2022 Mar 21;13(1):122. doi: 10.1186/s13287-022-02796-1. Stem Cell Res Ther. 2022. PMID: 35313959 Free PMC article. Clinical Trial. - The Broad Immunomodulatory Effects of IL-7 and Its Application In Vaccines.
Huang J, Long Z, Jia R, Wang M, Zhu D, Liu M, Chen S, Zhao X, Yang Q, Wu Y, Zhang S, Tian B, Mao S, Ou X, Sun D, Gao Q, Cheng A. Huang J, et al. Front Immunol. 2021 Dec 10;12:680442. doi: 10.3389/fimmu.2021.680442. eCollection 2021. Front Immunol. 2021. PMID: 34956167 Free PMC article. Review. - FOXO3 regulates the CD8 T cell response to a chronic viral infection.
Sullivan JA, Kim EH, Plisch EH, Suresh M. Sullivan JA, et al. J Virol. 2012 Sep;86(17):9025-34. doi: 10.1128/JVI.00942-12. Epub 2012 Jun 6. J Virol. 2012. PMID: 22675000 Free PMC article. - T cell exhaustion.
Wherry EJ. Wherry EJ. Nat Immunol. 2011 Jun;12(6):492-9. doi: 10.1038/ni.2035. Nat Immunol. 2011. PMID: 21739672 Review. - Innate and Adaptive Immune Regulation During Chronic Viral Infections.
Zuniga EI, Macal M, Lewis GM, Harker JA. Zuniga EI, et al. Annu Rev Virol. 2015 Nov;2(1):573-97. doi: 10.1146/annurev-virology-100114-055226. Epub 2015 Sep 2. Annu Rev Virol. 2015. PMID: 26958929 Free PMC article. Review.
References
- Virgin HW, Wherry EJ, Ahmed R. Redefining chronic viral infection. Cell. 2009;138(1):30–50. - PubMed
- Day CL, Kaufmann DE, Kiepiela P, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443(7109):350–354. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI59804/AI/NIAID NIH HHS/United States
- R21 AI068841/AI/NIAID NIH HHS/United States
- AI48785/AI/NIAID NIH HHS/United States
- R01 AI059804/AI/NIAID NIH HHS/United States
- AI68841/AI/NIAID NIH HHS/United States
- R21 AI048785/AI/NIAID NIH HHS/United States
- R01 AI048785/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous