Identification of contractile vacuole proteins in Trypanosoma cruzi - PubMed (original) (raw)
. 2011 Mar 18;6(3):e18013.
doi: 10.1371/journal.pone.0018013.
Veronica Jimenez, Miyoung Park, Vicente P Martins, James Atwood 3rd, Kristen Moles, Dalis Collins, Peter Rohloff, Rick Tarleton, Silvia N J Moreno, Ron Orlando, Roberto Docampo
Affiliations
- PMID: 21437209
- PMCID: PMC3060929
- DOI: 10.1371/journal.pone.0018013
Identification of contractile vacuole proteins in Trypanosoma cruzi
Paul N Ulrich et al. PLoS One. 2011.
Abstract
Contractile vacuole complexes are critical components of cell volume regulation and have been shown to have other functional roles in several free-living protists. However, very little is known about the functions of the contractile vacuole complex of the parasite Trypanosoma cruzi, the etiologic agent of Chagas disease, other than a role in osmoregulation. Identification of the protein composition of these organelles is important for understanding their physiological roles. We applied a combined proteomic and bioinfomatic approach to identify proteins localized to the contractile vacuole. Proteomic analysis of a T. cruzi fraction enriched for contractile vacuoles and analyzed by one-dimensional gel electrophoresis and LC-MS/MS resulted in the addition of 109 newly detected proteins to the group of expressed proteins of epimastigotes. We also identified different peptides that map to at least 39 members of the dispersed gene family 1 (DGF-1) providing evidence that many members of this family are simultaneously expressed in epimastigotes. Of the proteins present in the fraction we selected several homologues with known localizations in contractile vacuoles of other organisms and others that we expected to be present in these vacuoles on the basis of their potential roles. We determined the localization of each by expression as GFP-fusion proteins or with specific antibodies. Six of these putative proteins (Rab11, Rab32, AP180, ATPase subunit B, VAMP1, and phosphate transporter) predominantly localized to the vacuole bladder. TcSNARE2.1, TcSNARE2.2, and calmodulin localized to the spongiome. Calmodulin was also cytosolic. Our results demonstrate the utility of combining subcellular fractionation, proteomic analysis, and bioinformatic approaches for localization of organellar proteins that are difficult to detect with whole cell methodologies. The CV localization of the proteins investigated revealed potential novel roles of these organelles in phosphate metabolism and provided information on the potential participation of adaptor protein complexes in their biogenesis.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
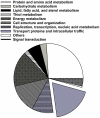
Figure 1. Annotated proteins in the contractile vacuole proteome belong to a variety of metabolic groups.
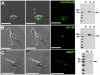
Figure 2. Fluorescence microscopy and western blot analysis of V-H+-ATPase subunit B-, AP180-, and VAMP1-GFP fusion proteins in live T. cruzi epimastigotes.
V-H+-ATPase subunit B (A), AP180 (B), and VAMP1 (C) localize to the bladder under hyposmotic conditions. Brightness and contrast of panels was adjusted, and fluorescence images in C were deconvolved. Scale bars: 10 µm. Confirmation of tagging by western blot analyses with polyclonal anti-GFP (dilution 1∶5,000-1∶10,000, Invitrogen) in epimastigotes. HRP-conjugated goat anti-rabbit was used as a secondary antibody. Magic Mark XP (Invitrogen) was used as a molecular weight marker. Arrows indicate bands of interest. A, V-H+-ATPase subunit B, expected size of fusion protein = 82 kDa. B, AP-180, expected size of fusion protein = 81 kDa. A 100 kDa cross-reacting band is only detected in the supernatant. C, VAMP1 expected size = 52 kDa. P, membrane pellet,S, soluble fraction, H, homogenate of whole parasites, WT, wild-type epimastigotes (negative control).
Figure 3. AP180 and SNARE 2.1 immuno-electron microscopy.
GFP fusion proteins were detected in epimastigotes with anti-GFP polyclonal antibodies and gold-conjugated anti-rabbit secondary antibody. AP180 localizes mainly in the bladder of the CV (A and B) while SNARE 2.1 clearly localizes in the vesicular structures of the spongiome (Cand D) although, some labeling can be observed is Golgi-like structures (arrow in D). CV: contractile vacuole bladder; S: spongiome; K: kinetoplast; N: nucleus. Bars: 0.5 µm.
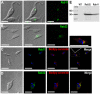
Figure 4. Rab-GFP fusion proteins localize in T. cruzi contractile vacuole.
Rab11 (green) (A) land Rab32 (green) (B) localize to the bladder under hyposmotic conditions. Rab11 (C) and Rab32 (D) partially co-localize with BODIPY-ceramide (red). DNA is stained with DAPI (blue). Brightness and contrast of panels was adjusted, and fluorescence images inC-D were deconvolved. Inset in C shows one cell (dotted rectangle) at higher magnification. Scale bars:A, B and C = 10 µm; D = 5 µm. E, confirmation of tagging by western blot analyses with anti GFP shows the expected size for both fusion proteins (50 kDa). Wild-type epimastigotes were used as negative control (WT).
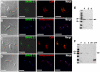
Figure 5. SNARE-GFP fusion proteins localize in the contractile vacuole spongiome.
SNARE2.1-GFP co-localizes with calmodulin (CaM) (A) and BODIPY-ceramide (B). SNARE2.2-GFP co-localizes with CaM (C) but localizes to a compartment that does not stain with BODIPY-ceramide (D). DNA is stained with DAPI (blue). Brightness and contrast of panels was adjusted, and fluorescence images in B and D were deconvolved. Scale bars = 10 µm. E, F, western blot analyses reveal the expected size for GFP tagged SNARE proteins (50 kDa). P, membrane pellet; S, soluble fraction, H, homogenate of whole parasites;WT, wild-type epimastigotes (negative control);GFP, epimastigotes overexpressing GFP (positive control).
Figure 6. CaM- and TcPho1-GFP fusion proteins localization.
CaM-GFP overexpressing parasites (A) showed a localized signal to the contractile vacuole (green) but also cytosolic distribution. This localization was confirmed with anti-GFP (A, in red). A monoclonal antibody against human CaM was used to perform IFA (B). Green corresponds to the CaM-GFP overexpressed protein and in red the specific localization for the anti-human CaM can be observed in the spongiome of the CV. TcPho1 is localized at the bladder of the CV (C) both by direct GFP signal (green) and labeled by anti-GFP (red). Under hyposmotic conditions (D, hypo), TcPho1 bladder localization becomes more evident. The expected molecular weight for fusion proteins is 44 kDa for CaM (E) and 107 kDa for TcPho1 (F).P, membrane pellet; S, soluble fraction;H, homogenate whole parasites; WT, wild-type epimastigotes (negative control); GFP, epimastigotes overexpressing GFP (positive control).
Similar articles
- Acidocalcisomes and the contractile vacuole complex are involved in osmoregulation in Trypanosoma cruzi.
Rohloff P, Montalvetti A, Docampo R. Rohloff P, et al. J Biol Chem. 2004 Dec 10;279(50):52270-81. doi: 10.1074/jbc.M410372200. Epub 2004 Oct 4. J Biol Chem. 2004. PMID: 15466463 - A functional aquaporin co-localizes with the vacuolar proton pyrophosphatase to acidocalcisomes and the contractile vacuole complex of Trypanosoma cruzi.
Montalvetti A, Rohloff P, Docampo R. Montalvetti A, et al. J Biol Chem. 2004 Sep 10;279(37):38673-82. doi: 10.1074/jbc.M406304200. Epub 2004 Jul 12. J Biol Chem. 2004. PMID: 15252016 - Rab11 regulates trafficking of trans-sialidase to the plasma membrane through the contractile vacuole complex of Trypanosoma cruzi.
Niyogi S, Mucci J, Campetella O, Docampo R. Niyogi S, et al. PLoS Pathog. 2014 Jun 26;10(6):e1004224. doi: 10.1371/journal.ppat.1004224. eCollection 2014 Jun. PLoS Pathog. 2014. PMID: 24968013 Free PMC article. - New insights into roles of acidocalcisomes and contractile vacuole complex in osmoregulation in protists.
Docampo R, Jimenez V, Lander N, Li ZH, Niyogi S. Docampo R, et al. Int Rev Cell Mol Biol. 2013;305:69-113. doi: 10.1016/B978-0-12-407695-2.00002-0. Int Rev Cell Mol Biol. 2013. PMID: 23890380 Free PMC article. Review. - The old and the new about the contractile vacuole of Trypanosoma cruzi.
Jimenez V, Miranda K, Augusto I. Jimenez V, et al. J Eukaryot Microbiol. 2022 Nov;69(6):e12939. doi: 10.1111/jeu.12939. Epub 2022 Aug 16. J Eukaryot Microbiol. 2022. PMID: 35916682 Free PMC article. Review.
Cited by
- An Overview of Trypanosoma cruzi Biology Through the Lens of Proteomics: A Review.
Telleria J, Costales JA. Telleria J, et al. Pathogens. 2025 Mar 31;14(4):337. doi: 10.3390/pathogens14040337. Pathogens. 2025. PMID: 40333120 Free PMC article. Review. - Frog-killing chytrid fungi deploy different strategies to regulate intracellular pressure in cell types that have or lack a cell wall.
Prostak S, Velle KB, Fritz-Laylin LK. Prostak S, et al. bioRxiv [Preprint]. 2025 May 14:2025.05.13.653819. doi: 10.1101/2025.05.13.653819. bioRxiv. 2025. PMID: 40462924 Free PMC article. Preprint. - A phosphoproteomic approach towards the understanding of the role of TGF-β in Trypanosoma cruzi biology.
Ferrão PM, de Oliveira FL, Degrave WM, Araujo-Jorge TC, Mendonça-Lima L, Waghabi MC. Ferrão PM, et al. PLoS One. 2012;7(6):e38736. doi: 10.1371/journal.pone.0038736. Epub 2012 Jun 12. PLoS One. 2012. PMID: 22719930 Free PMC article. - A conserved pressure-driven mechanism for regulating cytosolic osmolarity.
Velle KB, Garner RM, Beckford TK, Weeda M, Liu C, Kennard AS, Edwards M, Fritz-Laylin LK. Velle KB, et al. bioRxiv [Preprint]. 2023 Mar 2:2023.03.01.529730. doi: 10.1101/2023.03.01.529730. bioRxiv. 2023. PMID: 36909496 Free PMC article. Updated. Preprint. - Calcium signaling in trypanosomatid parasites.
Docampo R, Huang G. Docampo R, et al. Cell Calcium. 2015 Mar;57(3):194-202. doi: 10.1016/j.ceca.2014.10.015. Epub 2014 Nov 6. Cell Calcium. 2015. PMID: 25468729 Free PMC article. Review.
References
- Urbina JA, Docampo R. Specific chemotherapy of Chagas disease: controversies and advances. Trends Parasitol. 2003;19:495–501. - PubMed
- Kollien AH, Grospietsch T, Kleffmann T, Zerbst-Boroffka I, Schaub GA. Ionic composition of the rectal contents and excreta of the reduviid bug Triatoma infestans. . J Insect Physiol. 2001;47:739–747. - PubMed
- Lang F. Mechanisms and significance of cell volume regulation. J Am Coll Nutr. 2007;26:613S–623S. - PubMed
- Rohloff P, Montalvetti A, Docampo R. Acidocalcisomes and the contractile vacuole complex are involved in osmoregulation in Trypanosoma cruzi. . J Biol Chem. 2004;279:52270–52281. - PubMed
- Montalvetti A, Rohloff P, Docampo R. A functional aquaporin co-localizes with the vacuolar proton pyrophosphatase to acidocalcisomes and the contractile vacuole complex of Trypanosoma cruzi. J Biol Chem. 2004;279:38673–38682. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases