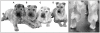A novel unstable duplication upstream of HAS2 predisposes to a breed-defining skin phenotype and a periodic fever syndrome in Chinese Shar-Pei dogs - PubMed (original) (raw)
. 2011 Mar;7(3):e1001332.
doi: 10.1371/journal.pgen.1001332. Epub 2011 Mar 17.
Jennifer R S Meadows, Katarina Truvé, Gerli Rosengren Pielberg, Francesca Puppo, Evan Mauceli, Javier Quilez, Noriko Tonomura, Giordana Zanna, Maria José Docampo, Anna Bassols, Anne C Avery, Elinor K Karlsson, Anne Thomas, Daniel L Kastner, Erik Bongcam-Rudloff, Matthew T Webster, Armand Sanchez, Ake Hedhammar, Elaine F Remmers, Leif Andersson, Lluis Ferrer, Linda Tintle, Kerstin Lindblad-Toh
Affiliations
- PMID: 21437276
- PMCID: PMC3060080
- DOI: 10.1371/journal.pgen.1001332
A novel unstable duplication upstream of HAS2 predisposes to a breed-defining skin phenotype and a periodic fever syndrome in Chinese Shar-Pei dogs
Mia Olsson et al. PLoS Genet. 2011 Mar.
Abstract
Hereditary periodic fever syndromes are characterized by recurrent episodes of fever and inflammation with no known pathogenic or autoimmune cause. In humans, several genes have been implicated in this group of diseases, but the majority of cases remain unexplained. A similar periodic fever syndrome is relatively frequent in the Chinese Shar-Pei breed of dogs. In the western world, Shar-Pei have been strongly selected for a distinctive thick and heavily folded skin. In this study, a mutation affecting both these traits was identified. Using genome-wide SNP analysis of Shar-Pei and other breeds, the strongest signal of a breed-specific selective sweep was located on chromosome 13. The same region also harbored the strongest genome-wide association (GWA) signal for susceptibility to the periodic fever syndrome (p(raw) = 2.3 × 10⁻⁶, p(genome) = 0.01). Dense targeted resequencing revealed two partially overlapping duplications, 14.3 Kb and 16.1 Kb in size, unique to Shar-Pei and upstream of the Hyaluronic Acid Synthase 2 (HAS2) gene. HAS2 encodes the rate-limiting enzyme synthesizing hyaluronan (HA), a major component of the skin. HA is up-regulated and accumulates in the thickened skin of Shar-Pei. A high copy number of the 16.1 Kb duplication was associated with an increased expression of HAS2 as well as the periodic fever syndrome (p < 0.0001). When fragmented, HA can act as a trigger of the innate immune system and stimulate sterile fever and inflammation. The strong selection for the skin phenotype therefore appears to enrich for a pleiotropic mutation predisposing these dogs to a periodic fever syndrome. The identification of HA as a major risk factor for this canine disease raises the potential of this glycosaminoglycan as a risk factor for human periodic fevers and as an important driver of chronic inflammation.
Conflict of interest statement
The authors KL-T, MO, and LT have filed a patent for development of a genetic test.
Figures
Figure 1. The phenotypic spectrum of the Chinese Shar-Pei.
Following strong selection for the “wrinkled” skin phenotype, Shar-Pei dogs in the western world most commonly present as the meatmouth type (A–C). The traditional type of Shar-Pei (D) is the ancestral version and is still common in China. The characteristic skin is a result of a deposition of mucin, mainly hyaluronic acid (HA), in the upper dermis of the skin. The deposit collects in certain areas of Shar-Pei skin and often as “socks” around the hocks (E). The meatmouth Shar-Pei (A–C) is also predisposed to a breed-specific periodic fever syndrome called Familial Shar-Pei Fever (FSF).
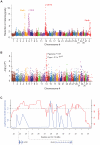
Figure 2. The association with Shar-Pei Fever susceptibility and the strongest selective sweep signal co-localize on chromosome 13.
(A) A 10-fold reduction of heterozygosity was identified on chromosome 13 when comparing Shar-Pei (n = 50) to 24 other canine breeds (n = 230). 50,000 SNPs were used to screen the whole genome using a sliding window approach (see Materials and Methods). (B) A case-control genome-wide association analysis identified a strong peak with several SNPs on chromosome 13 to be in association with Familial Shar-Pei Fever (FSF). After correcting for stratification and multiple testing (100,000 permutations), four SNPs retained significant association (p<0.05; strongest SNP association, CanFam 2.0 chr13: 27,913,803 Mb). Shar-Pei dogs used in the study were strictly classified into groups of affected (n = 22) and unaffected (n = 17) by FSF. (C) SNPs associated with FSF (blue line) are interspersed with the signals of selection (red line). The 39 Shar-Pei and 17,227 SNP common to both analyses were used to generate this graph.
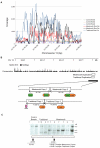
Figure 3. The identification of two breed-specific duplications in Shar-Pei.
(A) Targeted resequencing of a 1.5 Mb region on chromosome 13 identified a duplication with on average 3.5–4.5X higher read coverage in two meatmouth Shar-Pei (black and red), compared to three control breeds (green, Standard Poodle; orange, Neapolitan Mastiff and purple, Pug). A shorter duplication was detected in the traditional Shar-Pei (blue). (B) The meatmouth duplication was determined to be 16.1 Kb long (CanFam 2.0 Chr13: 23,746,089–23,762,189) with both breakpoints located in repeats (a SINE and a LINE) and with an insertion of 7 bp separating different copies. The duplication in the traditional Shar-Pei overlapped the meatmouth duplication and was slightly shorter, 14.3 Kb long (CanFam 2.0 Chr13: 23,743,906–23,758,214). In this case the copies were separated by 1 bp but were still anchored in repeat motifs (c) Southern blot analysis using _Bsr_GI digested gDNA from Shar-Pei and control breeds confirmed the existence of two duplication types in Shar-Pei. One meatmouth dog (lane 6) contained both duplication types. Individuals were classified as healthy (h) or as affected by Familial Shar-Pei Fever (f).
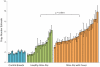
Figure 4. The relationship between copy number estimate and susceptibility to Familial Shar-Pei Fever.
A significant correlation (p = <0.0001, Mann Whitney test) was seen when the meatmouth copy number in unaffected Shar-Pei (n = 16, H+) and individuals affected by FSF (n = 28, FSF+ and FSF+ A) were compared. Based on this limited sample size, most dogs with more than six copies had fever whereas most dogs with less than four copies did not.
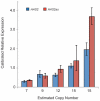
Figure 5. Expression analysis reveals a trend of increased HAS2 and HAS2as expression with copy number.
Expression levels were measured in dermal fibroblasts that were cultured from individual Shar-Pei skin biopsies. The individual with the lowest copy number (CNV = 5) was used to calibrate each assay.
Similar articles
- Hereditary cutaneous mucinosis in shar pei dogs is associated with increased hyaluronan synthase-2 mRNA transcription by cultured dermal fibroblasts.
Zanna G, Docampo MJ, Fondevila D, Bardagí M, Bassols A, Ferrer L. Zanna G, et al. Vet Dermatol. 2009 Oct;20(5-6):377-82. doi: 10.1111/j.1365-3164.2009.00799.x. Vet Dermatol. 2009. PMID: 20178474 - Increased HAS2-driven hyaluronic acid synthesis in shar-pei dogs with hereditary cutaneous hyaluronosis (mucinosis).
Docampo MJ, Zanna G, Fondevila D, Cabrera J, López-Iglesias C, Carvalho A, Cerrato S, Ferrer L, Bassols A. Docampo MJ, et al. Vet Dermatol. 2011 Dec;22(6):535-45. doi: 10.1111/j.1365-3164.2011.00986.x. Epub 2011 Jul 1. Vet Dermatol. 2011. PMID: 21718367 - A study of Shar-Pei dogs refutes association of the 'meatmouth' duplication near HAS2 with Familial Shar-Pei Fever.
Metzger J, Distl O. Metzger J, et al. Anim Genet. 2014 Oct;45(5):763-4. doi: 10.1111/age.12193. Epub 2014 Jul 5. Anim Genet. 2014. PMID: 25040095 No abstract available. - Skin diseases of the Chinese Shar-Pei.
Muller GH. Muller GH. Vet Clin North Am Small Anim Pract. 1990 Nov;20(6):1655-70. doi: 10.1016/s0195-5616(90)50166-7. Vet Clin North Am Small Anim Pract. 1990. PMID: 2251744 Review. - Insights into morphology and disease from the dog genome project.
Schoenebeck JJ, Ostrander EA. Schoenebeck JJ, et al. Annu Rev Cell Dev Biol. 2014;30:535-60. doi: 10.1146/annurev-cellbio-100913-012927. Epub 2014 Jul 9. Annu Rev Cell Dev Biol. 2014. PMID: 25062362 Free PMC article. Review.
Cited by
- Multiple FGF4 Retrocopies Recently Derived within Canids.
Batcher K, Dickinson P, Maciejczyk K, Brzeski K, Rasouliha SH, Letko A, Drögemüller C, Leeb T, Bannasch D. Batcher K, et al. Genes (Basel). 2020 Jul 23;11(8):839. doi: 10.3390/genes11080839. Genes (Basel). 2020. PMID: 32717834 Free PMC article. - Hyaluronidase facilitated subcutaneous immunoglobulin in primary immunodeficiency.
Jolles S. Jolles S. Immunotargets Ther. 2013 Sep 18;2:125-33. doi: 10.2147/ITT.S31136. eCollection 2013. Immunotargets Ther. 2013. PMID: 27471693 Free PMC article. Review. - An Improved microRNA Annotation of the Canine Genome.
Penso-Dolfin L, Swofford R, Johnson J, Alföldi J, Lindblad-Toh K, Swarbreck D, Moxon S, Di Palma F. Penso-Dolfin L, et al. PLoS One. 2016 Apr 27;11(4):e0153453. doi: 10.1371/journal.pone.0153453. eCollection 2016. PLoS One. 2016. PMID: 27119849 Free PMC article. - Characterization of Copy Number Variation's Potential Role in Marek's Disease.
Xu L, He Y, Ding Y, Sun G, Carrillo JA, Li Y, Ghaly MM, Ma L, Zhang H, Liu GE, Song J. Xu L, et al. Int J Mol Sci. 2017 May 9;18(5):1020. doi: 10.3390/ijms18051020. Int J Mol Sci. 2017. PMID: 28486430 Free PMC article. - Similar genomic proportions of copy number variation within gray wolves and modern dog breeds inferred from whole genome sequencing.
Serres-Armero A, Povolotskaya IS, Quilez J, Ramirez O, Santpere G, Kuderna LFK, Hernandez-Rodriguez J, Fernandez-Callejo M, Gomez-Sanchez D, Freedman AH, Fan Z, Novembre J, Navarro A, Boyko A, Wayne R, Vilà C, Lorente-Galdos B, Marques-Bonet T. Serres-Armero A, et al. BMC Genomics. 2017 Dec 19;18(1):977. doi: 10.1186/s12864-017-4318-x. BMC Genomics. 2017. PMID: 29258433 Free PMC article.
References
- Fraser JR, Laurent TC, Laurent UB. Hyaluronan: its nature, distribution, functions and turnover. Journal of Internal Medicine. 1997;242:27–33. - PubMed
- Wheeler-Jones CP, Farrar CE, Pitsillides AA. Targeting hyaluronan of the endothelial glycocalyx for therapeutic intervention. Current Opinion in Investigational Drugs. 2010;11(9):997–1006. - PubMed
- Laurent TC, Fraser JRE. Hyaluronan. FASEB J. 1992;6:2397–2404. - PubMed
- Zanna G, Fondevila D, Bardagi M, Docampo MJ, Bassols A, et al. Cutaneous mucinosis in shar-pei dogs is due to hyaluronic acid deposition and is associated with high levels of hyaluronic acid in serum. Vet Dermatol. 2008;19:314–318. - PubMed
- Ramsden CA, Bankier A, Brown TJ, Cowen PSJ, Frost GI. A new disorder of hyaluronan metabolism associated with generalized folding and thickening of the skin. J of Ped. 2000;36:62–68. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical