Neutrophil interactions with sialyl Lewis X on human nonsmall cell lung carcinoma cells regulate invasive behavior - PubMed (original) (raw)
. 2011 Oct 1;117(19):4493-505.
doi: 10.1002/cncr.26059. Epub 2011 Mar 22.
Affiliations
- PMID: 21437888
- PMCID: PMC3134589
- DOI: 10.1002/cncr.26059
Neutrophil interactions with sialyl Lewis X on human nonsmall cell lung carcinoma cells regulate invasive behavior
Catherine A St Hill et al. Cancer. 2011.
Abstract
Background: The carbohydrate sialyl Lewis X (sLeX) is expressed on leukocytes and carcinoma cells and binds to selectins during inflammatory processes and early metastasis. Synthesis of sLeX depends on activity of enzymes, including α(1,3/1,4) fucosyltransferase (FucT-III). Tumor necrosis factor-α (TNF-α) up-regulates FucT-III, resulting in increased sLeX in the airways of patients with respiratory disease; however, the mechanisms that regulate sLeX in the inflammatory tumor microenvironment are not well understood.
Methods: The authors stably transfected human lung carcinoma cell lines with the FucT-III gene and exposed them to TNF-α to investigate its role in regulation of sLeX expression and selectin-binding ability using semiquantitative real-time polymerase chain reaction and flow cytometry. Cytokine expression was examined in transfected cells using chemiluminescent arrays and enzyme-linked immunosorbent assays, and invasion was studied using Matrigel assays and alterations in morphology. Human lung tissue arrays were analyzed for immunohistochemical detection of sLeX and neutrophils.
Results: Stimulation of FucT-III-transfected cells with recombinant human (rh) TNF-α up-regulated sLeX expression and increased E-selectin binding. Transfected cells secreted high levels of interleukin 8, growth-regulated oncogene-α, and mast cell proteinase-1. Cells exposed to rhTNF-α, neutrophil-conditioned media, and cultures with a 5:1 ratio of neutrophils to cancer cells had significantly increased sLeX expression and invasiveness and underwent nonadherent morphologic changes. In lung carcinomas, but not in normal lung tissues, 71% of tumors were highly positive for sLeX expression in areas of increased neutrophil infiltration.
Conclusions: The current results indicated that neutrophils may be recruited to areas of FucT-III activity and sLeX expression in lung carcinomas to enhance the invasive and metastatic potential of lung cancer cells.
Copyright © 2011 American Cancer Society.
Figures
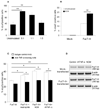
Figure 1. H1299 α(1,3/1,4) fucosyltransferase (FucT-III) transfected lung carcinoma cells express sLeX and bind to E-selectin
(A) FucT-III transfected cells express 2-fold higher mRNA levels of FucT-III than mock-transfected cells by reverse transcriptase-polymerase chain reaction. Experiments were repeated 5 times and 1 representative example is shown. Band intensities were normalized to β-actin by densitometry (bp indicates base pairs). (B) The percentage of sLeX positive cells is increased after FucT-III transfection and is up-regulated further after stimulation with 5 ng/ml rhTNF-α for 48 hours. Significant differences are observed between un-stimulated, mock-transfected cells and un-stimulated, FucT-III transfected cells, (double asterisks; p < 0.01) and between un-stimulated, FucT-III transfected cells and cells stimulated with rhTNF-α (single asterisk; p < 0.05). (C) FucT-III transfected H1299 cells substantially bind to E-selectin in the presence of calcium ions (Ca2+) compared to mock-transfected cells(double asterisk; p < 0.01), and binding is further enhanced after stimulation with rhTNF-α, (asterisk; p < 0.05) compared with un-stimulated, transfected cells. EDTA indicates ethylene diamine tetraacetic acid. Mean values of up to 5 experiments are shown.
Figure 2. H1299 cells transfected with α(1,3/1,4) fucosyltransferase (FucT-III) secrete neutrophil chemoattractants
(A) FucT-III–transfected cells secrete high levels of growth-regulated oncogene-alpha (GRO-α), interleukin-8 (IL-8), and mast cell proteinase-1 (MCP-1) but decreased levels of granulocyte macrophage–colony-stimulating factor (GM-CSF) and MCP-3 compared with un-manipulated cells (asterisks; p < 0.05). GCSF indicates granulocyte–colony-stimulating factor; MIG, monokine induced by interferon-c; RANTES, regulated upon activation, normal T-cell expressed and secreted; TGF-β, transforming growth factor-β; TNF-α, tumor necrosis factor-α. (B) FucT-III–transfected H1299 cells secreted 2-fold higher levels of IL-8 than mock-transfected cells when quantified by an enzyme-linked immunosorbent assay (asterisk; p < 0.05). The average of 2 independent experiments is shown and each experiment was repeated in duplicate.
Figure 3. Tumor necrosis factor-α (TNF-α) up-regulates sialyl Lewis X (sLeX) expression
(A) At ratios of 5:1 and 1:1 of neutrophils to FucT-III–transfected cells in coculture, significantly higher percentages of cells were positive for sLeX compared with un-stimulated cells (double asterisks; p < 0.01). (B) A significantly higher percentage of α (1,3/1,4) fucosyltransferase (FucT-III)-transfected cells were positive for sLeX after culture with neutrophil-conditioned media (NCM) compared with unstimulated, FucT-III–transfected cells (double asterisks; p < 0.01). (C) Increased percentages of sLeX-positive cells were observed when FucT-III–transfected H1299 cells treated with an isotype control monoclonal antibody (mAb) were cultured with a 1:1 ratio of neutrophils or NCM (single asterisk; p < 0.05), or 5 ng/mL recombinant human TNF-α (rhTNF-α) (double asterisks; p < 0.01) for 48 hours. In the presence of an anti-TNF-α –blocking mAb, sLeX-positive cells were down-regulated compared with control mAb-treated cells when cultured with neutrophils, NCM, or rhTNF-α (single asterisk; p < 0.05). Mean values from 5 experiments are shown for A through C. (D) FucT-III messenger RNA levels are unchanged in H1299 mock-transfected and FucT-III–transfected cells after stimulation with rhTNF-α or NCM for 48 hours. A representative experiment with 2 repetitions is shown (bp indicates base pairs).
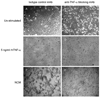
Figure 4. H1299 cells cultured with neutrophils have TNF-α-dependent morphological alterations
Photomicrographs depicting the morphology of (A) H1299 mock-transfected cells and (B) FucT-III transfected cells under un-stimulated conditions are shown. The appearance of (C) H1299 mock-transfected cells cultured for 48 hours with a 1:1 ratio of neutrophils to cancer cells resembles that of un-stimulated cells; however (D), FucT-III transfected cells under the same conditions as in (C) form viable, non-adherent aggregates of elongated, dendritic-like cells. H1299 mock-transfected cells cultured with neutrophils and an anti-TNF-α function blocking mAb (E) are morphologically similar to the cells in (A) and (C), whereas (F) FucT-III transfected cells under the same conditions as in (E) revert to a morphology that is similar to un-stimulated cells shown in (B) (original magnification, 100X in A-F). FucT-III transfected cells that were cultured with CFSE-labeled neutrophils are seen under (G) bright-field microscopy and (H) fluorescent microscopy (original magnification, 100X). Neutrophils are not present in the aggregates of cancer cells. Representative images from 3 independent experiments are shown.
Figure 5. H1299 α(1,3/1,4) fucosyltransferase (FucT-III)-transfected cells cultured with recombinant human tumor necrosis factor-α (rhTNF-α) or neutrophil-conditioned media (NCM) have TNF-α–dependent morphologic alterations
The morphology of unstimulated FucT-III–transfected H1299 cells cultured with (A) an isotype-matched control monoclonal antibody (mAb) or (B) or with an anti-TNF-α function-blocking mAb is similar. (C) Control mAb-treated cells that were cultured with 5 ng/mL rhTNF-a became elongated but reverted back to the appearance of un-stimulated cells after exposure to 10 μg/mL of an anti-TNF-α function-blocking mAb (D). (E) Transfected cells exposed to NCM formed aggregates of elongated cells, and this morphology was reversible after treatment with anti-TNF-α mAb (F). A representative experiment from 3 repetitions is shown (original magnification, 100X).
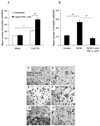
Figure 6. The invasiveness of H1299 α(1,3/1,4) fucosyltransferase (FucT-III)-transfected cells stimulated with recombinant human tumor necrosis factor-α (rhTNF-α) or neutrophil-conditioned media (NCM) is up-regulated
(A,E) Un-stimulated, H1299 FucT-III–transfected cells are more invasive than unstimulated, mock-transfected cells (C) after 24 hours in Matrigel assays (single asterisk; p < 0.05). The invasiveness of FucT-III–transfected cells (A,F), but not of mock-transfected cells (A,D), is up-regulated further after exposure to 5 ng/mL rhTNF-a (double asterisks; p < 0.01; A). (B,G) Exposure to NCM increases the invasiveness of FucT-III–transfected cells compared with un-stimulated, FucT-III transfected cells (E), and treatment with an anti-TNF-a monoclonal antibody (H) abrogates this effect compared with cells that were cultured with NCM alone (double asterisks; p < 0.01; B). Mean values of 3 experiments are shown in A and B, and representative photomicrographs of cells are shown in C–H (original magnification, 100X).
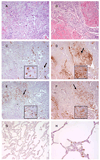
Figure 7. Neutrophils are recruited to sialyl Lewis X (sLeX)-positive areas of human lung carcinomas
These are representative photomicrographs of serial sections from (A,C,E) a lung adenocarcinoma and (B,D,F) a squamous cell carcinoma (original magnification, 200X). Insets show the areas of tissue indicated by the arrows (original magnification, 400X). Sections were stained with (A,B) H&E, (C,D) CSLEX-1 monoclonal antibody (mAb), and (E,F) neutrophil elastase antibody. Positively stained cells are brown. (G) This normal lung tissue sample was stained with the CSLEX-1 mAb (original magnification, 200X). Note the absence of brown color indicating positive staining. (H) This normal lung tissue sample was stained with neutrophil elastase antibody (original magnification, 400X). Positively stained neutrophils are brown.
Similar articles
- Carcinoembryonic antigen is a sialyl Lewis x/a carrier and an E‑selectin ligand in non‑small cell lung cancer.
Ferreira IG, Carrascal M, Mineiro AG, Bugalho A, Borralho P, Silva Z, Dall'olio F, Videira PA. Ferreira IG, et al. Int J Oncol. 2019 Nov;55(5):1033-1048. doi: 10.3892/ijo.2019.4886. Epub 2019 Sep 26. Int J Oncol. 2019. PMID: 31793656 Free PMC article. - A potential role for 6-sulfo sialyl Lewis X in metastasis of bladder urothelial carcinoma.
Taga M, Hoshino H, Low S, Imamura Y, Ito H, Yokoyama O, Kobayashi M. Taga M, et al. Urol Oncol. 2015 Nov;33(11):496.e1-9. doi: 10.1016/j.urolonc.2015.05.026. Epub 2015 Jun 29. Urol Oncol. 2015. PMID: 26137907 - C2-O-sLeX glycoproteins are E-selectin ligands that regulate invasion of human colon and hepatic carcinoma cells.
St Hill CA, Baharo-Hassan D, Farooqui M. St Hill CA, et al. PLoS One. 2011 Jan 19;6(1):e16281. doi: 10.1371/journal.pone.0016281. PLoS One. 2011. PMID: 21283832 Free PMC article. - Studies on selectin-carbohydrate interactions.
Jacob GS, Welply JK, Scudder PR, Kirmaier C, Abbas SZ, Howard SC, Keene JL, Schmuke JJ, Broschat K, Steininger C. Jacob GS, et al. Adv Exp Med Biol. 1995;376:283-90. doi: 10.1007/978-1-4615-1885-3_31. Adv Exp Med Biol. 1995. PMID: 8597260 Review. - Molecular mechanism for cancer-associated induction of sialyl Lewis X and sialyl Lewis A expression-The Warburg effect revisited.
Kannagi R. Kannagi R. Glycoconj J. 2004;20(5):353-64. doi: 10.1023/B:GLYC.0000033631.35357.41. Glycoconj J. 2004. PMID: 15229399 Review.
Cited by
- Trichomonas vaginalis triggers neutrophil extracellular traps reducing parasite integrity and growth.
Ramírez-Ledesma MG, Romero-Contreras YJ, Rodríguez MC, Reyes-Cortes R, Cuéllar-Mata P, Avila EE. Ramírez-Ledesma MG, et al. Parasitol Res. 2022 May;121(5):1355-1367. doi: 10.1007/s00436-022-07475-x. Epub 2022 Mar 8. Parasitol Res. 2022. PMID: 35258690 - Knockdown of α2,3-Sialyltransferases Impairs Pancreatic Cancer Cell Migration, Invasion and E-selectin-Dependent Adhesion.
Guerrero PE, Miró L, Wong BS, Massaguer A, Martínez-Bosch N, Llorens R, Navarro P, Konstantopoulos K, Llop E, Peracaula R. Guerrero PE, et al. Int J Mol Sci. 2020 Aug 28;21(17):6239. doi: 10.3390/ijms21176239. Int J Mol Sci. 2020. PMID: 32872308 Free PMC article. - FUT4 is involved in PD-1-related immunosuppression and leads to worse survival in patients with operable lung adenocarcinoma.
Liu C, Li Z, Wang S, Fan Y, Zhang S, Yang X, Hou K, Tong J, Hu X, Shi X, Wang X, Liu Y, Che X, Qu X. Liu C, et al. J Cancer Res Clin Oncol. 2019 Jan;145(1):65-76. doi: 10.1007/s00432-018-2761-y. Epub 2018 Oct 24. J Cancer Res Clin Oncol. 2019. PMID: 30357521 - Selectins in cancer immunity.
Borsig L. Borsig L. Glycobiology. 2018 Sep 1;28(9):648-655. doi: 10.1093/glycob/cwx105. Glycobiology. 2018. PMID: 29272415 Free PMC article. Review. - Antibody repertoire profiling with mimotope arrays.
Pashova S, Schneider C, von Gunten S, Pashov A. Pashova S, et al. Hum Vaccin Immunother. 2017 Feb;13(2):314-322. doi: 10.1080/21645515.2017.1264786. Epub 2016 Dec 8. Hum Vaccin Immunother. 2017. PMID: 27929733 Free PMC article.
References
- Coello MC, Luketich JD, Litle VR, et al. Prognostic significance of micrometastasis in non-small-cell lung cancer. Clin Lung Cancer. 2004;5:214–225. - PubMed
- Nakamori S, Kameyama M, Imaoka S, et al. Increased expression of sialyl Lewisx antigen correlates with poor survival in patients with colorectal carcinoma: clinicopathological and immunohistochemical study. Cancer Res. 1993;53:3632–3637. - PubMed
- Izumi Y, Kawamura YJ, Irimura T. Carbohydrate antigens in carcinoma invasion and metastasis. Nippon Geka Gakkai Zasshi. 1996;97:140–144. - PubMed
- Ogawa JI, Inoue H, Koide S. alpha-2,3-Sialyltransferase type 3N and alpha-1,3-fucosyltransferase type VII are related to sialyl Lewis(x) synthesis and patient survival from lung carcinoma. Cancer. 1997;79:1678–1685. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical