The core FOXG1 syndrome phenotype consists of postnatal microcephaly, severe mental retardation, absent language, dyskinesia, and corpus callosum hypogenesis - PubMed (original) (raw)
Case Reports
. 2011 Jun;48(6):396-406.
doi: 10.1136/jmg.2010.087528. Epub 2011 Mar 25.
Soma Das, Max Flindt, Deborah J Morris-Rosendahl, Irina Stefanova, Amy Goldstein, Denise Horn, Eva Klopocki, Gerhard Kluger, Peter Martin, Anita Rauch, Agathe Roumer, Sulagna Saitta, Laurence E Walsh, Dagmar Wieczorek, Gökhan Uyanik, Kerstin Kutsche, William B Dobyns
Affiliations
- PMID: 21441262
- PMCID: PMC5522617
- DOI: 10.1136/jmg.2010.087528
Case Reports
The core FOXG1 syndrome phenotype consists of postnatal microcephaly, severe mental retardation, absent language, dyskinesia, and corpus callosum hypogenesis
Fanny Kortüm et al. J Med Genet. 2011 Jun.
Abstract
Background: Submicroscopic deletions in 14q12 spanning FOXG1 or intragenic mutations have been reported in patients with a developmental disorder described as a congenital variant of Rett syndrome. This study aimed to further characterise and delineate the phenotype of FOXG1 mutation positive patients.
Method: The study mapped the breakpoints of a 2;14 translocation by fluorescence in situ hybridisation and analysed three chromosome rearrangements in 14q12 by cytogenetic analysis and/or array comparative genomic hybridisation. The FOXG1 gene was sequenced in 210 patients, including 129 patients with unexplained developmental disorders and 81 MECP2 mutation negative individuals.
Results: One known mutation, seen in two patients, and nine novel mutations of FOXG1 including two deletions, two chromosome rearrangements disrupting or displacing putative cis-regulatory elements from FOXG1, and seven sequence changes, are reported. Analysis of 11 patients in this study, and a further 15 patients reported in the literature, demonstrates a complex constellation of features including mild postnatal growth deficiency, severe postnatal microcephaly, severe mental retardation with absent language development, deficient social reciprocity resembling autism, combined stereotypies and frank dyskinesias, epilepsy, poor sleep patterns, irritability in infancy, unexplained episodes of crying, recurrent aspiration, and gastro-oesophageal reflux. Brain imaging studies reveal simplified gyral pattern and reduced white matter volume in the frontal lobes, corpus callosum hypogenesis, and variable mild frontal pachgyria.
Conclusions: These findings have significantly expanded the number of FOXG1 mutations and identified two affecting possible cis-regulatory elements. While the phenotype of the patients overlaps both classic and congenital Rett syndrome, extensive clinical evaluation demonstrates a distinctive and clinically recognisable phenotype which the authors suggest designating as the FOXG1 syndrome.
Conflict of interest statement
Figures
Figure 1
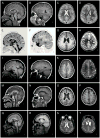
Brain imaging in a normal 3-year-old girl (A–D) and four patients with FOXG1 mutations (E–T). Here and in figure 2, each row shows four images from the same patient. The columns contain midline sagittal (left column) and parasagittal (2nd column) images, and axial images through the lateral ventricles (3rd column) and high convexity (right column). All four patients have a low forehead that reflects underlying microcephaly, foreshortened frontal lobes and reduced white matter volume that appears severe in the frontal lobes (asterisks in 12/16 images) and subtle in posterior regions. All four have hypogenesis of the anterior corpus callosum with a “pointed hook” appearance in three of them (arrows in E, M, Q) that consists of striking narrowing of the anterior body, genu and rostrum with the tiny rostrum forming a pointed tip, and relatively normal posterior callosum. In the remaining patient (arrow in I), the entire body of the corpus callosum is narrow and the genu small but less so than the others, leaving it dysmorphic but different from the “pointed hook” appearance. Three patients have subtle pachygyria over the frontal lobes only that consists of mildly short and wide gyri and mildly thick cortex (arrowheads in F–H, J–L, N–P), a subtle abnormality that may be accentuated by the reduced volume of the white matter. The increased cortical thickness was not seen in the remaining patients (R–T and Figure 2). Also, three patients had mildly enlarged lateral ventricles, with one having abnormally narrow anterior body and frontal horns (S). These images come from patients 1 (E–H), 5 (I–L), 7 (M–P) and 9 (Q–T), and a normal control (A–D).
Figure 2
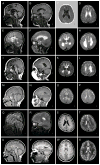
Brain imaging in another six patients with FOXG1 mutations. Five have mildly enlarged lateral ventricles. The patients in the top four rows have changes similar to those seen in Figure 1, including microcephaly (E, I, M; not obvious in A, Q), short frontal lobes and reduced volume of frontal white matter (asterisks in 8/16 images). Hypogenesis of the corpus callosum was seen in 4/5, but with different patterns. The first patient (A) has a thin and dysmorphic corpus callosum similar to Figure 1I, the next total agenesis of the corpus callosum (E), and the third and fourth partial agenesis with a short and thin corpus callosum (I, M). No patients in this group have pachygyria. The last two patients have normal brain imaging except for subtle reduced volume of the frontal white matter and mildly simplified gyral pattern. These images come from patients 6 (A–D), 3 (E–H), 2 (I–L), 10 (M–P), 4 (Q–T), and 11 (U–X).
Figure 3
Deletions including FOXG1 and regulatory mutations. (A) Physical map of the 14q12-q13.1 region. Genes in this region are represented by arrows indicating the 5’ 3’ orientation. Fosmid clones (F- [G248P8]; WIBR-2 Human Fosmid Library) used for mapping the 14q12 breakpoint of the 2;14 translocation in patient 1 (1) are indicated by colored bars and names are given. Color code of fosmids; black: mapped distal to the translocation breakpoint; blue: spanned the breakpoint; green: mapped proximal to breakpoint. The breakpoint region is indicated by a wavy vertical arrow in red. (B) Deletions in 14q12. Horizontal black lines depict the deletions identified in patients 2 – 4 (2, 3, 4). Arrowheads at the left and right side of the deletion in patient 2 show that this deletion extends to either side. Dotted lines indicate that the respective deletion breakpoints have not been fine-mapped. BAC clones (RP11 Human BAC Library) used for FISH analysis to confirm the presence of the deletions in the patients are indicated by red bars and names are given. (C) A detailed view of the region containing putative long-range _cis_-regulatory elements of FOXG1. In the upper part, the two black vertical bars represent potential regulatory elements (genomic positions chr14:28,754,647-28,756,495 bp and 28,930,280-28,932,099 bp) that have been identified with the ESPERR (evolutionary and sequence pattern extraction through reduced representations) computational method. In the lower part, a more detailed view of the region of high Regulatory Potential (RP) values, with the UCSC genome browser map of seven-way (human, chimpanzee, macaque, mouse, rat, dog, and cow; March 2006 assembly) RP analysis of the candidate _cis_-regulatory elements distal to FOXG1.
Figure 4
Patients with FOXG1 mutations. Two patients with 14q12 deletions (top row, A–B) demonstrate mild facial dysmorphism consisting of round face with flat midface, low nasal bridge, bulbous nasal tip, thin upper lip and prominent ears (A–B). Four children with regulatory (top row, C and bottom row, D) or intragenic mutations (bottom row, E–F) have normal facial appearance except for mildly prominent ears. One photo shows hand stereotypy (D), and another shows striking esotropia (F). These patient numbers and mutations are shown at the bottom of each photo.
Similar articles
- 14q12 and severe Rett-like phenotypes: new clinical insights and physical mapping of FOXG1-regulatory elements.
Allou L, Lambert L, Amsallem D, Bieth E, Edery P, Destrée A, Rivier F, Amor D, Thompson E, Nicholl J, Harbord M, Nemos C, Saunier A, Moustaïne A, Vigouroux A, Jonveaux P, Philippe C. Allou L, et al. Eur J Hum Genet. 2012 Dec;20(12):1216-23. doi: 10.1038/ejhg.2012.127. Epub 2012 Jun 27. Eur J Hum Genet. 2012. PMID: 22739344 Free PMC article. - 14q12 microdeletions excluding FOXG1 give rise to a congenital variant Rett syndrome-like phenotype.
Ellaway CJ, Ho G, Bettella E, Knapman A, Collins F, Hackett A, McKenzie F, Darmanian A, Peters GB, Fagan K, Christodoulou J. Ellaway CJ, et al. Eur J Hum Genet. 2013 May;21(5):522-7. doi: 10.1038/ejhg.2012.208. Epub 2012 Sep 12. Eur J Hum Genet. 2013. PMID: 22968132 Free PMC article. - Revisiting the phenotype associated with FOXG1 mutations: two novel cases of congenital Rett variant.
Bahi-Buisson N, Nectoux J, Girard B, Van Esch H, De Ravel T, Boddaert N, Plouin P, Rio M, Fichou Y, Chelly J, Bienvenu T. Bahi-Buisson N, et al. Neurogenetics. 2010 May;11(2):241-9. doi: 10.1007/s10048-009-0220-2. Epub 2009 Oct 6. Neurogenetics. 2010. PMID: 19806373 - A FOXG1 mutation in a boy with congenital variant of Rett syndrome.
Le Guen T, Bahi-Buisson N, Nectoux J, Boddaert N, Fichou Y, Diebold B, Desguerre I, Raqbi F, Daire VC, Chelly J, Bienvenu T. Le Guen T, et al. Neurogenetics. 2011 Feb;12(1):1-8. doi: 10.1007/s10048-010-0255-4. Epub 2010 Aug 24. Neurogenetics. 2011. PMID: 20734096 Review. - Thin genu of the corpus callosum points to mutation in FOXG1 in a child with acquired microcephaly, trigonocephaly, and intellectual developmental disorder: a case report and review of literature.
De Bruyn C, Vanderhasselt T, Tanyalçin I, Keymolen K, Van Rompaey KL, De Meirleir L, Jansen AC. De Bruyn C, et al. Eur J Paediatr Neurol. 2014 May;18(3):420-6. doi: 10.1016/j.ejpn.2013.11.010. Epub 2013 Dec 6. Eur J Paediatr Neurol. 2014. PMID: 24388699 Review.
Cited by
- Genotyping FOXG1 Mutations in Patients with Clinical Evidence of the FOXG1 Syndrome.
Pratt DW, Warner JV, Williams MG. Pratt DW, et al. Mol Syndromol. 2013 Jan;3(6):284-7. doi: 10.1159/000345845. Epub 2012 Dec 12. Mol Syndromol. 2013. PMID: 23599699 Free PMC article. - MEF2C Haploinsufficiency features consistent hyperkinesis, variable epilepsy, and has a role in dorsal and ventral neuronal developmental pathways.
Paciorkowski AR, Traylor RN, Rosenfeld JA, Hoover JM, Harris CJ, Winter S, Lacassie Y, Bialer M, Lamb AN, Schultz RA, Berry-Kravis E, Porter BE, Falk M, Venkat A, Vanzo RJ, Cohen JS, Fatemi A, Dobyns WB, Shaffer LG, Ballif BC, Marsh ED. Paciorkowski AR, et al. Neurogenetics. 2013 May;14(2):99-111. doi: 10.1007/s10048-013-0356-y. Epub 2013 Feb 7. Neurogenetics. 2013. PMID: 23389741 Free PMC article. - Delineating FOXG1 syndrome: From congenital microcephaly to hyperkinetic encephalopathy.
Vegas N, Cavallin M, Maillard C, Boddaert N, Toulouse J, Schaefer E, Lerman-Sagie T, Lev D, Magalie B, Moutton S, Haan E, Isidor B, Heron D, Milh M, Rondeau S, Michot C, Valence S, Wagner S, Hully M, Mignot C, Masurel A, Datta A, Odent S, Nizon M, Lazaro L, Vincent M, Cogné B, Guerrot AM, Arpin S, Pedespan JM, Caubel I, Pontier B, Troude B, Rivier F, Philippe C, Bienvenu T, Spitz MA, Bery A, Bahi-Buisson N. Vegas N, et al. Neurol Genet. 2018 Nov 7;4(6):e281. doi: 10.1212/NXG.0000000000000281. eCollection 2018 Dec. Neurol Genet. 2018. PMID: 30533527 Free PMC article. - Discordant congenital Zika syndrome twins show differential in vitro viral susceptibility of neural progenitor cells.
Caires-Júnior LC, Goulart E, Melo US, Araujo BHS, Alvizi L, Soares-Schanoski A, de Oliveira DF, Kobayashi GS, Griesi-Oliveira K, Musso CM, Amaral MS, daSilva LF, Astray RM, Suárez-Patiño SF, Ventini DC, Gomes da Silva S, Yamamoto GL, Ezquina S, Naslavsky MS, Telles-Silva KA, Weinmann K, van der Linden V, van der Linden H, de Oliveira JRM, Arrais NMR, Melo A, Figueiredo T, Santos S, Meira JGC, Passos SD, de Almeida RP, Bispo AJB, Cavalheiro EA, Kalil J, Cunha-Neto E, Nakaya H, Andreata-Santos R, de Souza Ferreira LC, Verjovski-Almeida S, Ho PL, Passos-Bueno MR, Zatz M. Caires-Júnior LC, et al. Nat Commun. 2018 Feb 2;9(1):475. doi: 10.1038/s41467-017-02790-9. Nat Commun. 2018. PMID: 29396410 Free PMC article. - In vivo epigenetic editing of Sema6a promoter reverses transcallosal dysconnectivity caused by C11orf46/Arl14ep risk gene.
Peter CJ, Saito A, Hasegawa Y, Tanaka Y, Nagpal M, Perez G, Alway E, Espeso-Gil S, Fayyad T, Ratner C, Dincer A, Gupta A, Devi L, Pappas JG, Lalonde FM, Butman JA, Han JC, Akbarian S, Kamiya A. Peter CJ, et al. Nat Commun. 2019 Sep 11;10(1):4112. doi: 10.1038/s41467-019-12013-y. Nat Commun. 2019. PMID: 31511512 Free PMC article.
References
- Bisgaard AM, Kirchhoff M, Tumer Z, Jepsen B, Brondum-Nielsen K, Cohen M, Hamborg-Petersen B, Bryndorf T, Tommerup N, Skovby F. Additional chromosomal abnormalities in patients with a previously detected abnormal karyotype, mental retardation, and dysmorphic features. Am J Med Genet A. 2006;140:2180–7. - PubMed
- Mencarelli MA, Kleefstra T, Katzaki E, Papa FT, Cohen M, Pfundt R, Ariani F, Meloni I, Mari F, Renieri A. 14q12 Microdeletion syndrome and congenital variant of Rett syndrome. Eur J Med Genet. 2009;52:148–52. - PubMed
- Papa FT, Mencarelli MA, Caselli R, Katzaki E, Sampieri K, Meloni I, Ariani F, Longo I, Maggio A, Balestri P, Grosso S, Farnetani MA, Berardi R, Mari F, Renieri A. A 3 Mb deletion in 14q12 causes severe mental retardation, mild facial dysmorphisms and Rett-like features. Am J Med Genet A. 2008;146A:1994–8. - PubMed
- Shoichet SA, Kunde SA, Viertel P, Schell-Apacik C, von Voss H, Tommerup N, Ropers HH, Kalscheuer VM. Haploinsufficiency of novel FOXG1B variants in a patient with severe mental retardation, brain malformations and microcephaly. Hum Genet. 2005;117:536–44. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases