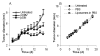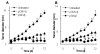Replicase-based plasmid DNA shows anti-tumor activity - PubMed (original) (raw)
Replicase-based plasmid DNA shows anti-tumor activity
B Leticia Rodriguez et al. BMC Cancer. 2011.
Abstract
Background: Double stranded RNA (dsRNA) has multiple anti-tumor mechanisms. Over the past several decades, there have been numerous attempts to utilize synthetic dsRNA to control tumor growth in animal models and clinical trials. Recently, it became clear that intracellular dsRNA is more effective than extracellular dsRNA on promoting apoptosis and orchestrating adaptive immune responses. To overcome the difficulty in delivering a large dose of synthetic dsRNA into tumors, we propose to deliver a RNA replicase-based plasmid DNA, hypothesizing that the dsRNA generated by the replicase-based plasmid in tumor cells will inhibit tumor growth.
Methods: The anti-tumor activity of a plasmid (pSIN-β) that encodes the sindbis RNA replicase genes (nsp1-4) was evaluated in mice with model tumors (TC-1 lung cancer cells or B16 melanoma cells) and compared to a traditional pCMV-β plasmid.
Results: In cell culture, transfection of tumor cells with pSIN-β generated dsRNA. In mice with model tumors, pSIN-β more effectively delayed tumor growth than pCMV-β, and in some cases, eradicated the tumors.
Conclusion: RNA replicase-based plasmid may be exploited to generate intracellular dsRNA to control tumor growth.
© 2011 Rodriguez et al; licensee BioMed Central Ltd.
Figures
Figure 1
A schematic of plasmids used in this study. CMV, cytomegalovirus promoter; Lac-Z, β-galactosidase; nsp, sindbis virus sequences coding for the nonstructural proteins (nsp1-4). pSIN-β-Δnsp (8,727 bp), pSIN-β (14,869 bp), pCMV-β (7,164 bp).
Figure 2
Generation of dsRNA in tumor cells transfected with pSIN-β. (A). RT-PCR confirmed the presence of sindbis virus nsp4 gene mRNA and its anti-sense strand in tumor cells transfected with pSIN-β. TC-1 cells were transfected with pCMV-β (pCMV) or pSin-β (pSIN), or left untreated (N/A). Total RNA was reverse transcribed into DNA with oligo dT primer or primers specific to the nsp4 gene (forward p4F or reverse p4R) before PCR amplification. This experiment was repeated twice with similar results. (B). ELISA confirmed the presence of an elevated level of dsRNA in TC-1 cells transfected with pSIN-β (n = 3). Total dsRNA was isolated from TC-1 cells transfected with pCMV-β or pSIN-β and used to coat ELISA plate. The primary Ab was the J2 anti-dsRNA IgG2a. *, p = 0.004. (C). Transfection of pSIN-β into TC-1 cells inhibited cell growth. TC-1 cells (20 000 cells/well) were transfected with the same amount (0.4 μg) of pCMV-β, pSIN-β, or pSIN-β-Δnsp (n = 4). Cell numbers were quantified using MTT assay and normalized to cells treated with sterile PBS. Data shown are mean ± S.E.M. **, at 48 and 72 h, the value of the pSIN-β were different from that of the pCMV-β and the pSIN-β-Δnsp (p < 0.05).
Figure 3
In vivo expression of nsp4 gene. Twenty-four h after i.m. injection with PBS, pCMV-β, pSIN-β, or pSIN-β-Δnsp, total RNA was extracted from the muscle tissues and RT-PCR-amplified to detect the expression of nsp4 and β-gal genes.
Figure 4
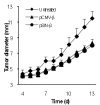
Treatment of mice with the pSIN-β caused TC-1 tumor regression. (A). C57BL/6 mice (n = 5) were s.c. implanted with TC-1 tumor cells (5 × 105) on day 0. DNA-liposome lipoplexes were injected (s.c., p.t.) for 10 consecutive days, starting on day 5 (25 μg DNA per day). (*) indicates that on days 13-15 the values of pCMV-β and pSIN-β were different from each other (p < 0.05). (B). Peritumoral injection of liposomes alone or sterile PBS did not affect the growth of the TC-1 tumors. Mice (n = 5) with TC-1 tumors were injected (p.t.) with sterile PBS or liposomes in PBS (dose equivalent to that injected in the DNA-liposome lipoplexes) for 10 consecutive days, starting on day 4. Data shown were mean ± S.E.M.
Figure 5
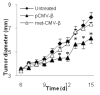
Deletion of the replicase genes (nsp1-3 and part of nsp4) from the pSIN-β plasmid significantly decreased the anti-tumor activity of the plasmid. C57BL/6 mice (n = 4-5) were s.c. implanted with TC-1 tumor cells (5 × 105) on day 0. From days 4 to 13, mice were injected (s.c., p.t.) with lipoplexes prepared with pSIN-β (25 μg) or pSIN-β-Δnsp (25 μg). *, On day 8, p = 0.05, pSIN-β vs. pSIN-β-Δnsp.
Figure 6
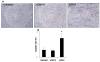
Injection with pSIN-β promoted more tumor cells to undergo apoptosis. (A). Micrographs of tumors stained against anti-caspase-3 (brown). (B). Apoptotic index. Data shown were mean ± S.E.M. The number of mice in each group was 3-4. (*) Indicates that the value of pSIN-β differed from that of the others (ANOVA, p = 0.03).
Figure 7
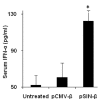
The pSIN-β plasmid induced IFN-α production in mouse sera. IFN-α levels in blood were measured 10 h after injection (n = 4). Data reported are means ± SEM. (*, p < 0.05, pCMV-β vs. pSIN-β).
Figure 8
The pSIN-β plasmid was no longer more effective than pCMV-β against tumors in athymic mice. Mice (n = 6-8) were s.c. implanted with TC-1 tumor cells (5 × 105) on day 0. From days 4 to 13, mice were injected (s.c., p.t) with lipoplexes prepared with pSIN-β (25 μg) or pCMV-β (25 μg).
Figure 9
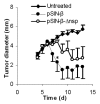
Unmethylated CpG motifs contributed to the anti-tumor activity of the pCMV-β. C57BL/6 mice (n = 5-6) were implanted with TC-1 tumor cells (5 × 105) on day 0. From days 6 to 15, mice were injected (s.c., p.t.) with lipoplexes prepared with unmethylated or methylated pCMV-β (pCMV-β or met-CMV-β, 25 μg). Data shown were mean ± S.E.M. (*) indicates that on days 11 to 15, the values of pCMV-β and met-CMV-β were different from each other (p < 0.05).
Figure 10
pSIN-β was more effective than pCMV-β in controlling the growth of mouse B16-F10 and B16-OVA melanomas as well. C57BL/6 mice (n = 6-7) were implanted with B16-F10 ( A) or B16-OVA (B) cells on day 0. DNA-liposome lipoplexes were injected (s.c., p.t.) for 10 consecutive days starting on day 3 (25 μg DNA per day). (*) indicate that on days 9-12 for B16-F10 (or days 9-11 for B16-OVA), the values of pCMV-β and pSIN-β were different from each other (p < 0.05). Data shown are mean ± S.E.M.
Similar articles
- Antitumor activity of tumor-targeted RNA replicase-based plasmid that expresses interleukin-2 in a murine melanoma model.
Rodriguez BL, Blando JM, Lansakara-P DS, Kiguchi Y, DiGiovanni J, Cui Z. Rodriguez BL, et al. Mol Pharm. 2013 Jun 3;10(6):2404-15. doi: 10.1021/mp400033m. Epub 2013 May 17. Mol Pharm. 2013. PMID: 23641783 Free PMC article. - Control of solid tumor growth in mice using EGF receptor-targeted RNA replicase-based plasmid DNA.
Rodriguez BL, Li X, Kiguchi K, DiGiovanni J, Unger EC, Cui Z. Rodriguez BL, et al. Nanomedicine (Lond). 2012 Apr;7(4):475-91. doi: 10.2217/nnm.11.112. Epub 2012 Feb 2. Nanomedicine (Lond). 2012. PMID: 22296186 Free PMC article. - Sequence and time dependence of transfection efficiency of electrically-assisted gene delivery to tumors in mice.
Cemazar M, Pavlin D, Kranjc S, Grosel A, Mesojednik S, Sersa G. Cemazar M, et al. Curr Drug Deliv. 2006 Jan;3(1):77-81. doi: 10.2174/156720106775197556. Curr Drug Deliv. 2006. PMID: 16472096 - Plasmid DNA electrotransfer: a new non viral method for gene therapy in oncology.
Bureau MF, Scherman D. Bureau MF, et al. Technol Cancer Res Treat. 2002 Apr;1(2):149-52. doi: 10.1177/153303460200100208. Technol Cancer Res Treat. 2002. PMID: 12622522 - Induction of antigen-specific immune responses against malignant brain tumors by intramuscular injection of sindbis DNA encoding gp100 and IL-18.
Yamanaka R, Xanthopoulos KG. Yamanaka R, et al. DNA Cell Biol. 2005 May;24(5):317-24. doi: 10.1089/dna.2005.24.317. DNA Cell Biol. 2005. PMID: 15869409
Cited by
- Antitumor activity of tumor-targeted RNA replicase-based plasmid that expresses interleukin-2 in a murine melanoma model.
Rodriguez BL, Blando JM, Lansakara-P DS, Kiguchi Y, DiGiovanni J, Cui Z. Rodriguez BL, et al. Mol Pharm. 2013 Jun 3;10(6):2404-15. doi: 10.1021/mp400033m. Epub 2013 May 17. Mol Pharm. 2013. PMID: 23641783 Free PMC article. - Recent Advances and Prospects of Nucleic Acid Therapeutics for Anti-Cancer Therapy.
Lee M, Lee M, Song Y, Kim S, Park N. Lee M, et al. Molecules. 2024 Oct 7;29(19):4737. doi: 10.3390/molecules29194737. Molecules. 2024. PMID: 39407665 Free PMC article. Review. - Control of solid tumor growth in mice using EGF receptor-targeted RNA replicase-based plasmid DNA.
Rodriguez BL, Li X, Kiguchi K, DiGiovanni J, Unger EC, Cui Z. Rodriguez BL, et al. Nanomedicine (Lond). 2012 Apr;7(4):475-91. doi: 10.2217/nnm.11.112. Epub 2012 Feb 2. Nanomedicine (Lond). 2012. PMID: 22296186 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous