N-acetylcysteine prevents loss of dopaminergic neurons in the EAAC1-/- mouse - PubMed (original) (raw)
N-acetylcysteine prevents loss of dopaminergic neurons in the EAAC1-/- mouse
Ari E Berman et al. Ann Neurol. 2011 Mar.
Abstract
Objective: Dopaminergic neuronal death in Parkinson's disease (PD) is accompanied by oxidative stress and preceded by glutathione depletion. The development of disease-modifying therapies for PD has been hindered by a paucity of animal models that mimic these features and demonstrate an age-related progression. The EAAC1(-/-) mouse may be useful in this regard, because EAAC1(-/-) mouse neurons have impaired neuronal cysteine uptake, resulting in reduced neuronal glutathione content and chronic oxidative stress. Here we aimed to (1) characterize the age-related changes in nigral dopaminergic neurons in the EAAC1(-/-) mouse, and (2) use the EAAC1(-/-) mouse to evaluate N-acetylcysteine, a membrane-permeable cysteine pro-drug, as a potential disease-modifying intervention for PD.
Methods: Wild-type mice, EAAC1(-/-) mice, and EAAC1(-/-) mice chronically treated with N-acetylcysteine were evaluated at serial time points for evidence of oxidative stress, dopaminergic cell death, and motor abnormalities.
Results: EAAC1(-/-) mice showed age-dependent loss of dopaminergic neurons in the substantia nigra pars compacta, with more than 40% of these neurons lost by age 12 months. This neuronal loss was accompanied by increased nitrotyrosine formation, nitrosylated α-synuclein, and microglial activation. These changes were substantially reduced in mice that received N-acetylcysteine.
Interpretation: These findings suggest that the EAAC1(-/-) mouse may be a useful model of the chronic neuronal oxidative stress that occurs in PD. The salutary effects of N-acetylcysteine in this mouse model provide an impetus for clinical evaluation of glutathione repletion in PD.
Copyright © 2010 American Neurological Association.
Conflict of interest statement
Potential Conflict of Interest
Nothing to report.
Figures
FIGURE 1
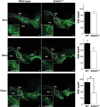
Progressive loss of SNc dopaminergic neurons in _EAAC1_−/− mice. Confocal images of the dorsal midbrain of WT and _EAAC1_−/− mice, with dopaminergic neurons stained green (anti-TH). Insets show magnified view of boxed areas. Graphs show quantification of dopaminergic neurons in the SNc at 3, 9, and 12 months of age. Bar = 200 µm. **p < 0.01; n = 3–7. SNc = substantia nigra pars compacta; SNr = substantia nigra pars reticulata; VTA = ventral tegmental area; WT = wild-type.
FIGURE 2
Microglial activation in the SNc of _EAAC1_−/− mice. (A) Confocal images of the dorsal midbrain of WT and _EAAC1_−/− mice, with microglia stained green (anti-Iba1). Dopaminergic neurons were stained blue (anti-TH) to delineate the SNc. Right-hand figures show magnified view of microglia in the inset boxed areas. Bar = 100 µm in low-power view, 50µm in high-power view. (B) Quantification of microglial activation in the SNc. *p < 0.01; n = 3. SNc = substantia nigra pars compacta; SNr = substantia nigra pars reticulata; VTA = ventral tegmental area; WT = wild-type.
FIGURE 3
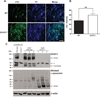
Increased oxidative stress in _EAAC1_−/− SNc dopaminergic neurons. (A) Confocal images of SNc from WT and _EAAC1_−/− mice. Sections are stained for nitrotyrosine (nTyr, green) as a marker of oxidative stress, and for tyrosine hydroxylase (TH, blue) to identify dopaminergic neurons. Bar = 50 µm. (B) Quantified data show that the nTyr signal localized to dopaminergic neurons is increased in _EAAC1_−/− mice. **p < 0.01; n = 7. (C) Western blots prepared from WT and EAAC1−/− mouse brain show accumulation of nitrated α-synuclein in 12-month-old EAAC1−/− brains but not in 12-month-old WT brains. The 2 left-hand lanes were prepared on a different gel than the other lanes. *Denotes a nonspecific band recognized by the antibody to EAAC1. WT = wild-type.
FIGURE 4
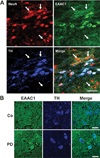
EAAC1 expression in mouse and human SNc dopaminergic neurons. (A) Sections through mouse SNc immunostained for NeuN (red) to identify neuronal nuclei; for EAAC1 (green); and for tyrosine hydroxylase (TH, blue) to identify dopaminergic neurons. Confocal images show dopaminergic neurons to be densely stained for EAAC1, and nondopaminergic neurons (some denoted by arrows) to be less densely stained. Bar = 50µm; representative of n = 4. (B) Sections through human normal and PD brains immunostained for EAAC1 (green), and for TH (blue) to identify dopaminergic neurons. The TH-positive dopaminergic neurons show coexpression of EAAC1 in both PD and control brains. Bar = 50µm; representative of n = 4 control and 4 PD brains.
FIGURE 5
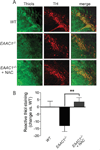
Oral N-acetylcysteine restores reactive thiol content of SNc dopaminergic neurons in _EAAC1_−/− mice. (A) Representative confocal sections through mouse SNc labeled with C5-maleimide (green) to label reactive thiols and immunostained for tyrosine hydroxylase (red) to identify dopaminergic neurons. Bar = 50µm. (B) Quantification of C5-maeimide fluorescence in TH-positive neurons indicates that reactive thiol content of dopaminergic neurons is lower in _EAAC1_−/− mice than in WT mice, but normalized in _EAAC1_−/− mice treated with oral NAC. *p < 0.05; n = 4.
FIGURE 6
N-acetylcysteine improves survival of SNc dopaminergic neurons in _EAAC1_−/− mice. (A) Images prepared as in Figure 1, with dopaminergic neurons stained green (anti-TH) and neuronal nuclei stained red (anti-NeuN). Neuronal loss in the SNc is reduced in mice treated with N-acetylcysteine (NAC). Bars = 200µm. (B) Cell counts of SNc dopaminergic neurons in _EAAC1_−/− mice with and without NAC treatment. **p < 0.01; n = 5–6. Note that the cell count data for _EAAC1_−/− mice without NAC treatment are the same as in Figure 1, reshown here to facilitate comparisons. (C) Sections are stained for nitrotyrosine (nTyr, green) as a marker of oxidative stress, and for tyrosine hydroxylase (TH, blue) to identify dopaminergic neurons. Bars = 50µm. (D) Quantified data show that the nTyr signal localized to dopaminergic neurons is reduced in the _EAAC1_−/− mice treated with NAC. **p < 0.01; n = 5–7.
FIGURE 7
N-acetyl-cysteine preserves motor function in _EAAC1_−/− mice. _EAAC1_−/− and wild-type (WT) mice were continuously treated with NAC-supplemented water (NAC) or normal water, and motor agility was evaluated by the pole test at ages 9 and 12 months. Y-axis shows the mean time required for mice to invert position and climb down the vertical pole. *p < 0.05; n = 7–10.
Similar articles
- Oxidative stress on EAAC1 is involved in MPTP-induced glutathione depletion and motor dysfunction.
Aoyama K, Matsumura N, Watabe M, Nakaki T. Aoyama K, et al. Eur J Neurosci. 2008 Jan;27(1):20-30. doi: 10.1111/j.1460-9568.2007.05979.x. Epub 2007 Dec 17. Eur J Neurosci. 2008. PMID: 18093171 - Neuronal glutathione deficiency and age-dependent neurodegeneration in the EAAC1 deficient mouse.
Aoyama K, Suh SW, Hamby AM, Liu J, Chan WY, Chen Y, Swanson RA. Aoyama K, et al. Nat Neurosci. 2006 Jan;9(1):119-26. doi: 10.1038/nn1609. Epub 2005 Nov 27. Nat Neurosci. 2006. PMID: 16311588 - EAAC1 gene deletion increases neuronal death and blood brain barrier disruption after transient cerebral ischemia in female mice.
Choi BY, Kim JH, Kim HJ, Lee BE, Kim IY, Sohn M, Suh SW. Choi BY, et al. Int J Mol Sci. 2014 Oct 27;15(11):19444-57. doi: 10.3390/ijms151119444. Int J Mol Sci. 2014. PMID: 25350110 Free PMC article. - Glial reactions in Parkinson's disease.
McGeer PL, McGeer EG. McGeer PL, et al. Mov Disord. 2008 Mar 15;23(4):474-83. doi: 10.1002/mds.21751. Mov Disord. 2008. PMID: 18044695 Review. - Age and Parkinson's disease-related neuronal death in the substantia nigra pars compacta.
Eriksen N, Stark AK, Pakkenberg B. Eriksen N, et al. J Neural Transm Suppl. 2009;(73):203-13. doi: 10.1007/978-3-211-92660-4_16. J Neural Transm Suppl. 2009. PMID: 20411779 Review.
Cited by
- Extremely Low Frequency Magnetic Field (ELF-MF) Exposure Sensitizes SH-SY5Y Cells to the Pro-Parkinson's Disease Toxin MPP(.).
Benassi B, Filomeni G, Montagna C, Merla C, Lopresto V, Pinto R, Marino C, Consales C. Benassi B, et al. Mol Neurobiol. 2016 Aug;53(6):4247-4260. doi: 10.1007/s12035-015-9354-4. Epub 2015 Jul 30. Mol Neurobiol. 2016. PMID: 26223801 - Antioxidant Therapies for Ulcerative Dermatitis: A Potential Model for Skin Picking Disorder.
George NM, Whitaker J, Vieira G, Geronimo JT, Bellinger DA, Fletcher CA, Garner JP. George NM, et al. PLoS One. 2015 Jul 13;10(7):e0132092. doi: 10.1371/journal.pone.0132092. eCollection 2015. PLoS One. 2015. PMID: 26167859 Free PMC article. - Group I mGluR-regulated translation of the neuronal glutamate transporter, excitatory amino acid carrier 1.
Ross JR, Ramakrishnan H, Porter BE, Robinson MB. Ross JR, et al. J Neurochem. 2011 Jun;117(5):812-23. doi: 10.1111/j.1471-4159.2011.07233.x. Epub 2011 Apr 11. J Neurochem. 2011. PMID: 21371038 Free PMC article. - Current trends and future prospects of N-acetylcysteine utilizations in Parkinson's disease: A literature network analysis.
Muthmainah M, Wiyono N, Syah FK, Purnianto A, Yudhani RD, Wasita B. Muthmainah M, et al. J Taibah Univ Med Sci. 2025 May 29;20(3):298-306. doi: 10.1016/j.jtumed.2025.05.004. eCollection 2025 Jun. J Taibah Univ Med Sci. 2025. PMID: 40510771 Free PMC article. Review. - Bilirubin Prevents the TH+ Dopaminergic Neuron Loss in a Parkinson's Disease Model by Acting on TNF-α.
Jayanti S, Moretti R, Tiribelli C, Gazzin S. Jayanti S, et al. Int J Mol Sci. 2022 Nov 17;23(22):14276. doi: 10.3390/ijms232214276. Int J Mol Sci. 2022. PMID: 36430754 Free PMC article.
References
- Betarbet R, Sherer TB, MacKenzie G, et al. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci. 2000;3:1301–1306. - PubMed
- Floor E, Wetzel MG. Increased protein oxidation in human substantia nigra pars compacta in comparison with basal ganglia and prefrontal cortex measured with an improved dinitrophenylhydrazine assay. J Neurochem. 1998;70:268–275. - PubMed
- Dexter DT, Sian J, Rose S, et al. Indices of oxidative stress and mitochondrial function in individuals with incidental Lewy body disease. Ann Neurol. 1994;35:38–44. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous