MicroRNAs 29 are involved in the improvement of ventricular compliance promoted by aerobic exercise training in rats - PubMed (original) (raw)
MicroRNAs 29 are involved in the improvement of ventricular compliance promoted by aerobic exercise training in rats
U P R Soci et al. Physiol Genomics. 2011.
Abstract
MiRNAs regulate cardiac development, hypertrophy, and angiogenesis, but their role in cardiac hypertrophy (CH) induced by aerobic training has not previously been studied. Aerobic training promotes physiological CH preserving cardiac function. This study assessed involvement of miRNAs-29 in CH of trained rats. Female Wistar rats (n=7/group) were randomized into three groups: sedentary (S), training 1 (T1), training 2 (T2). T1: swimming sessions of 60 min/5 days/wk/10 wk. T2: similar to T1 until 8th wk. On the 9th wk rats swam 2×/day, and on the 10th wk 3×/day. MiRNAs analysis was performed by miRNA microarray and confirmed by real-time PCR. We assessed: markers of training, CH by ratio of left ventricle (LV) weight/body wt and cardiomyocytes diameter, pathological markers of CH (ANF, skeletal α-actin, α/β-MHC), collagen I and III (COLIAI and COLIIIAI) by real-time PCR, protein collagen by hydroxyproline (OH-proline) concentration, CF and CH by echocardiography. Training improved aerobic capacity and induced CH. MiRNAs-1, 133a, and 133b were downregulated as observed in pathological CH, however, without pathological markers. MiRNA-29c expression increased in T1 (52%) and T2 (123%), correlated with a decrease in COLIAI and COLIIIAI expression in T1 (27%, 38%) and T2 (33%, 48%), respectively. MiRNA-29c was inversely correlated to OH-proline concentration (r=0.61, P<0.05). The E/A ratio increased in T2, indicating improved LV compliance. Thus, these results show that aerobic training increase miR-29 expression and decreased collagen gene expression and concentration in the heart, which is relevant to the improved LV compliance and beneficial cardiac effects, associated with aerobic high performance training.
Figures
Fig. 1.
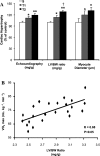
Effect of different aerobic exercise training volume on cardiac hypertrophy (CH) of Wistar rats. Data are presented as means ± SD. A: CH was displayed by echocardiography (mg/g), left ventricle (LV) weight-to-body weight ratio (LV/BW, mg/g) and cardiomyocyte diameter analysis in sedentary (S, n = 7) and trained groups (T1, n = 6; T2, n = 6). Significant difference vs. *S, †T1 P < 0.05; **T1, P < 0.01. B: the increase of CH was positively correlated with the increased of VO2max (S, n = 7; T1, n = 7; T2, n = 8) (r = 0.68, P < 0.05).
Fig. 2.
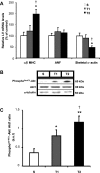
Effect of swimming exercise training on classical molecular markers of cardiac hypertrophy. A: α/β-myosin heavy chain (MHC) ratio, atrial natriuretic factor (ANF), and skeletal α-actin evaluated by real-time PCR. Targeted genes were normalized by cyclophilin mRNA. B: representative blots of cardiac phosphoSer473-Akt, Akt1, and α-tubulin from S, T1, and T2 groups. C: cardiac Akt activation (represented by phosphoSer473-Akt/Akt1 ratio). Targeted bands were normalized to cardiac α-tubulin. Groups: S (n = 7), T1 (n = 6), and T2 (n = 6). Data are reported as means ± SD. Significant difference vs. *S, †T1, P < 0.05; **S, P < 0.01.
Fig. 3.
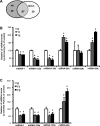
Differential expression of microRNAs (miRNAs) in LV induced by swimming exercise training. A: total of miRNAs differentially expressed by microarray: 87 miRNAs presented significant difference, 48 upregulated, 39 downregulated (P < 0.01 vs. *S). B: relative expression of miRNAs-1, 133a, 133b, 29a, 29b, and 29c related to S group by microarray (S, n = 2; T1, n = 2; T2, n = 2) (P < 0.01 vs. S). C: confirmation of differential expression of miRNAs-1, 133a, 133b, and 29c by real-time PCR reaction, percentage related to S group (%) S (n = 5), T1 (n = 5). and T2 (n = 5).
Fig. 4.
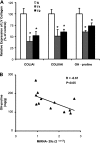
Gene and protein expression of collagen. A: collagen type I gene relative expression by real-time PCR, COLIAI (S, n = 5; T1, n = 5; T2, n = 5), collagen type III gene relative expression by real-time PCR, COLIIIAI (S, n = 5; T1, n = 5; T2, n = 5), LV collagen quantified from the hydroxyproline (OH-proline) concentration (mg/g) (S, n = 4; T1, n = 4; T2, n = 4). Values are expressed in percentage (%) from control group (Significant difference vs. *S, P < 0.05). B: the increase of miRNA-29c expression was negatively correlated with the decreased OH-proline concentration in LV (r = −0.61, P < 0.05). Significant difference vs.*S, P < 0.05.
Similar articles
- Swimming training in rats increases cardiac MicroRNA-126 expression and angiogenesis.
DA Silva ND Jr, Fernandes T, Soci UP, Monteiro AW, Phillips MI, DE Oliveira EM. DA Silva ND Jr, et al. Med Sci Sports Exerc. 2012 Aug;44(8):1453-62. doi: 10.1249/MSS.0b013e31824e8a36. Med Sci Sports Exerc. 2012. PMID: 22330028 - Anabolic steroids induce cardiac renin-angiotensin system and impair the beneficial effects of aerobic training in rats.
Rocha FL, Carmo EC, Roque FR, Hashimoto NY, Rossoni LV, Frimm C, Anéas I, Negrão CE, Krieger JE, Oliveira EM. Rocha FL, et al. Am J Physiol Heart Circ Physiol. 2007 Dec;293(6):H3575-83. doi: 10.1152/ajpheart.01251.2006. Epub 2007 Sep 28. Am J Physiol Heart Circ Physiol. 2007. PMID: 17906098 - mTOR signaling-related microRNAs as cardiac hypertrophy modulators in high-volume endurance training.
Pelozin BRA, Soci UPR, Gomes JLP, Oliveira EM, Fernandes T. Pelozin BRA, et al. J Appl Physiol (1985). 2022 Jan 1;132(1):126-139. doi: 10.1152/japplphysiol.00881.2020. Epub 2021 Nov 18. J Appl Physiol (1985). 2022. PMID: 34792404 - Regulation of cardiac microRNAs induced by aerobic exercise training during heart failure.
Souza RW, Fernandez GJ, Cunha JP, Piedade WP, Soares LC, Souza PA, de Campos DH, Okoshi K, Cicogna AC, Dal-Pai-Silva M, Carvalho RF. Souza RW, et al. Am J Physiol Heart Circ Physiol. 2015 Nov 15;309(10):H1629-41. doi: 10.1152/ajpheart.00941.2014. Epub 2015 Sep 25. Am J Physiol Heart Circ Physiol. 2015. PMID: 26408546 - Aerobic exercise training promotes physiological cardiac remodeling involving a set of microRNAs.
Fernandes T, Baraúna VG, Negrão CE, Phillips MI, Oliveira EM. Fernandes T, et al. Am J Physiol Heart Circ Physiol. 2015 Aug 15;309(4):H543-52. doi: 10.1152/ajpheart.00899.2014. Epub 2015 Jun 12. Am J Physiol Heart Circ Physiol. 2015. PMID: 26071549 Free PMC article. Review.
Cited by
- Downregulation of miR-26b-5p, miR-204-5p, and miR-497-3p Expression Facilitates Exercise-Induced Physiological Cardiac Hypertrophy by Augmenting Autophagy in Rats.
Qi J, Luo X, Ma Z, Zhang B, Li S, Zhang J. Qi J, et al. Front Genet. 2020 Feb 19;11:78. doi: 10.3389/fgene.2020.00078. eCollection 2020. Front Genet. 2020. PMID: 32140172 Free PMC article. - Exercise Training Attenuates Right Ventricular Remodeling in Rats with Pulmonary Arterial Stenosis.
de Melo BL, Vieira SS, Antônio EL, Dos Santos LF, Portes LA, Feliciano RS, de Oliveira HA, Silva JA Jr, de Carvalho PT, Tucci PJ, Serra AJ. de Melo BL, et al. Front Physiol. 2016 Dec 5;7:541. doi: 10.3389/fphys.2016.00541. eCollection 2016. Front Physiol. 2016. PMID: 27994552 Free PMC article. - The Role of MicroRNAs in the Cardiac Response to Exercise.
Liu X, Platt C, Rosenzweig A. Liu X, et al. Cold Spring Harb Perspect Med. 2017 Dec 1;7(12):a029850. doi: 10.1101/cshperspect.a029850. Cold Spring Harb Perspect Med. 2017. PMID: 28389519 Free PMC article. Review. - Resistance training attenuates salt overload-induced cardiac remodeling and diastolic dysfunction in normotensive rats.
Barretti DLM, Melo SFS, Oliveira EM, Barauna VG. Barretti DLM, et al. Braz J Med Biol Res. 2017 Aug 7;50(9):e6146. doi: 10.1590/1414-431X20176146. Braz J Med Biol Res. 2017. PMID: 28793051 Free PMC article. - Physical exercise and epigenetic adaptations of the cardiovascular system.
Zimmer P, Bloch W. Zimmer P, et al. Herz. 2015 May;40(3):353-60. doi: 10.1007/s00059-015-4213-7. Herz. 2015. PMID: 25744210 Review.
References
- Bergman I, Loxley R. New spectrophotometric method for the determination of proline in tissue hydrolyzates. Anal Chem 42: 702–706, 1970 - PubMed
- Carè A, Catalucci D, Felicetti F, Bonci D, Addario A, Gallo P, Bang ML, Segnalini P, Gu Y, Dalton ND, Elia L, Latronico MV, Høydal M, Autore C, Russo MA, Dorn GW, 2nd, Ellingsen O, Ruiz-Lozano P, Peterson KL, Croce CM, Peschle C, Condorelli G. MicroRNA-133 controls cardiac hypertrophy. Nat Med 13: 613–618, 2007 - PubMed
- Catalucci D, Gallo P, Condorelli G. MicroRNAs in cardiovascular biology and heart disease. Circ Cardiovasc Genet 2: 402–408, 2009 - PubMed
- Chen CH, Zhou YL, Wu YF, Cao Y, Gao JS, Tang JB. Effectiveness of microRNA in Down-regulation of TGF-beta gene expression in digital flexor tendons of chickens: in vitro and in vivo study. J Hand Surg Am 34: 1777–1784. e.1, 2009 - PubMed
- Chien KR, Knowlton KU, Zhu H, Chien S. Regulation of cardiac gene expression during myocardial growth and hypertrophy: molecular studies of an adaptive physiological response. FASEB J 5: 3037–3046, 1991 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials