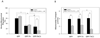Truncated tau and Aβ cooperatively impair mitochondria in primary neurons - PubMed (original) (raw)
Truncated tau and Aβ cooperatively impair mitochondria in primary neurons
Rodrigo A Quintanilla et al. Neurobiol Aging. 2012 Mar.
Abstract
Mitochondrial dysfunction is likely a significant contributing factor to Alzheimer disease pathogenesis, and both amyloid peptide (Aβ) and pathological forms of tau may contribute to this impairment. Cleavage of tau at Asp421 occurs early in Alzheimer disease, and Asp421-cleaved tau likely negatively impacts neuronal function. Previously we showed that expression of Asp421-cleaved tau in a neuronal cell model resulted in mitochondrial impairment. To extend these findings we expressed either full length tau or Asp421-cleaved tau (truncated tau) in primary cortical neurons and measured different aspects of mitochondrial function with or without the addition of sublethal concentrations of Aβ. The expression of truncated tau alone induced significant mitochondrial fragmentation in neurons. When truncated tau expression was combined with Aβ at sublethal concentrations, increases in the stationary mitochondrial population and the levels of oxidative stress in cortical neurons were observed. Truncated tau expression also enhanced Aβ-induced mitochondrial potential loss in primary neurons. These new findings show that Asp421-cleaved tau and Aβ cooperate to impair mitochondria, which likely contributes to the neuronal dysfunction in Alzheimer disease.
Copyright © 2012 Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosure statement
None of the authors has any actual or potential conflict of interest. Appropriate approval and procedures were used concerning animals.
Figures
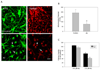
Figure 1. A low concentration of Aβ affects mitochondrial function in primary neurons
(A) Representative confocal images from primary cortical neurons that were loaded with Mitotracker Green (MTG) and TMRM to determine mitochondrial localization and membrane potential changes after exposure to 0.5 µM Aβ for 24 h. Aβ-treatment altered mitochondrial localization (increasing mitochondria accumulation, see white arrows) and significantly affected mitochondrial potential levels (TMRM fluorescence levels were decreased after treatment). Bar scale represents 10 µm. (B) Quantification of mitochondrial potential levels from cortical neurons treated with Aβ for 24 h. Graph represents quantification of mitochondrial potential fluorescence intensities as relative units, which shows that neurons treated with Aβ for 24 h exhibit a pronounced loss of mitochondrial potential. Data are mean ± S.E. (bars) from three separate experiments. *p < 0.05, compared to untreated cortical neurons. (C) Cell viability loss was evaluated using resazurin assay in primary cortical neurons treated with 0.5 or 2.5 µM Aβ for 24 or 48 h. No loss of viability was detected with 0.5µM Aβ, however 2.5 µM Aβ significantly reduced neuronal viability compared to untreated cells. Data are mean ± S.E. (bars). *, p < 0.01 compared to untreated neurons. * p < 0.01 by unpaired Student's t test.
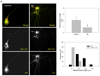
Figure 2. A low concentration of Aβ induces mitochondrial fragmentation in primary neurons
(A) Cortical neurons were transiently transfected with GFP/Mito-mCherry (Mito-mCh), and mitochondrial morphology and localization was observed prior to (control) and after 24 h treatment with 0.5 µM Aβ. Representative fluorescent images of untreated neurons showed elongated mitochondrial morphology with evident mitochondrial presence at all neuronal processes (see white arrows). In contrast, neurons treated with Aβ showed a significant accumulation of mitochondria in the neuronal soma (white arrows). Bar scale represents 10 µm. (B) Quantification of 3 different experiments revealed that mitochondria in Aβ-treated cells showed a significant reduction in average mitochondrial length as compared to mitochondria in untreated neurons. Data are mean ± S.E. (bars)*, p < 0.05 compare to untreated cells. * p < 0.05 was estimated by unpaired Student's t test. (C) Mitochondrial population was represented in terms of frequency of mitochondrial length present in cortical neurons positive transfected with GFP/Mito-mCherry and incubated in control conditions or with 0.5 µM Aβ for 24 h. Around 70% of mitochondria in neurons expressing GFP and treated with Aβ were less than 2 µm in length in comparison with untreated GFP positive neurons that the majority of mitochondria ranged between 2–4 µm.
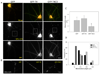
Figure 3. Effects of caspase-cleaved tau expression on mitochondrial morphology in primary neurons
(A) Primary cortical neurons were doubly transfected with GFP/Mito-mCherry (Mito-mCh), GFP-T4/Mito-mCh or GFP-T4C3/Mito-mCh and mitochondrial morphology was observed. Representative fluorescent images taken 48 h after transfection show mostly long and elongated mitochondrial morphology in neurons expressing GFP or GFP-T4. In contrast, expression of GFP-T4C3 resulted in significant mitochondrial fragmentation and reduced mitochondrial presence in the neuronal processes. Bar scale represents 10 µm. (B) Magnification of boxed regions from A to emphasize differences of mitochondrial morphology in neurites. Bar scale represents 5 µm. (C) Quantification of 4 independent experiments revealed that mitochondria in neurons expressing GFP-T4C3 showed more than a two-fold decrease in average mitochondrial length as compared to mitochondria in neurons expressing GFP-T4. Data are mean ± S.E. (bars)*, p < 0.05 compare to GFP-T4 untreated neurons. *p < 0.05 was estimated by unpaired Student's t test. (D) Mitochondrial population was represented in terms of frequency of mitochondrial length present in cortical neurons transfected with GFP, T4-GFP or GFP-T4C3. More than 60% of mitochondria in neurons expressing GFP-T4C3 were less than 2 µm in length while the majority of mitochondria in GFP and GFP-T4 neurons ranged between 2–6 µm. Quantification is from 4 independent experiments.
Figure 4. Caspase-cleaved tau expression enhances impairment of mitochondrial transport induced by Aβ in cortical neurons
(A) Primary cortical neurons were doubly transfected with GFP/Mito-mCh, GFP-T4/Mito-mCh, or GFP-T4C3/Mito-mCh to evaluate mitochondrial movement. Transfection with GFP-T4 and GFP-T4C3 significantly decreased the percentage of moving mitochondria in comparison with GFP-expressing neurons. (A) Quantification of mitochondrial moving population in neurons expressing GFP, GFP-T4 or GFP-T4C3 that were untreated (black bars) or treated with 0.5 µM Aβ (grey bars) for 2 h prior to measuring movement. Bars graph shows quantification of four independent experiments. Data are mean ± S.E. (bars). *, p < 0.05. p < 0.05 was estimated by unpaired Student's t test. (B) Mitochondrial transport velocity was evaluated in the moving mitochondria population in neurons transfected with GFP, GFP-T4 or GFP-T4C3 without (grey bars) or with pre-treatment with 0.5 µM Aβ bars) for 2 h. Untreated neurons showed similar mitochondrial transport rates. Interestingly, Aβ-treatment decreased mitochondrial transport velocity in GFP and GFP-T4 positive neurons, at the same manner. However, pretreatment with Aβ induced a more pronounced decrease in mitochondrial velocity in GFP-T4C3 expressing neurons. Bars graph shows quantification of four independent experiments. Data are mean ± S.E. (bars). *, p < 0.05. p < 0.05 was estimated by unpaired Student's t test.
Figure 5. Caspase-cleaved tau expression enhances mitochondrial impairment induced by Aβ treatment in primary neuronal cultures
(A) Primary cortical neurons were transfected with GFP, GFP-T4 or GFP-T4C3 and mitochondrial membrane potential was determined using TMRM. Treatment with 0.5 µM Aβ induced a significant mitochondrial potential loss in GFP-T4C3 cells. Untransfected, GFP, and GFP-T4 neurons showed a similar mild decrease in mitochondrial potential levels. The graph trends represent quantification of TMRM fluorescence intensities as relative units, from four independent experiments. (B) Quantification of mitochondrial potential levels after 30 min Aβ exposure. Data are mean ± S.E. (bars) from four separate experiments. *p < 0.01 compare to GFP-T4 cells treated with 0.5 µM Aβ. p < 0.01 was estimated by unpaired Student's t test.
Figure 6. Caspase-cleaved tau expression and Aβ treatment enhance superoxide production in primary neuronal cultures
(A) Primary cortical neurons were transfected with GFP, GFP-T4 or GFP-T4C3 and superoxide levels were determined using dihydroethidium (DHE) after treatment with 0.5 µM Aβ for 1 h. White arrows in the DHE panels indicate neurons that were transfected. Images show that superoxide levels in GFP and GFP-T4 neurons are very similar but in neurons transfected with GFP-T4C3, treatment with Aβ induced a significant increase in levels of superoxide. Bar scale represents 10 µm. (B) Quantification of three separate experiments revealed that caspase-cleaved tau expression in primary neurons significantly increased superoxide production induced by Aβ treatment. Data are mean ± S.E. (bars). *p < 0.01 compare to GFP-T4 cells treated with 0.5 µM Aβ. p < 0.01 was estimated by unpaired Student's t test.
Similar articles
- The Role of Mitochondrial Impairment in Alzheimer´s Disease Neurodegeneration: The Tau Connection.
Quntanilla RA, Tapia-Monsalves C. Quntanilla RA, et al. Curr Neuropharmacol. 2020;18(11):1076-1091. doi: 10.2174/1570159X18666200525020259. Curr Neuropharmacol. 2020. PMID: 32448104 Free PMC article. Review. - Caspase cleavage of tau: linking amyloid and neurofibrillary tangles in Alzheimer's disease.
Gamblin TC, Chen F, Zambrano A, Abraha A, Lagalwar S, Guillozet AL, Lu M, Fu Y, Garcia-Sierra F, LaPointe N, Miller R, Berry RW, Binder LI, Cryns VL. Gamblin TC, et al. Proc Natl Acad Sci U S A. 2003 Aug 19;100(17):10032-7. doi: 10.1073/pnas.1630428100. Epub 2003 Jul 29. Proc Natl Acad Sci U S A. 2003. PMID: 12888622 Free PMC article. - Caspase-Cleaved Tau Impairs Mitochondrial Dynamics in Alzheimer's Disease.
Pérez MJ, Vergara-Pulgar K, Jara C, Cabezas-Opazo F, Quintanilla RA. Pérez MJ, et al. Mol Neurobiol. 2018 Feb;55(2):1004-1018. doi: 10.1007/s12035-017-0385-x. Epub 2017 Jan 13. Mol Neurobiol. 2018. PMID: 28084594 - Tau facilitates Aβ-induced loss of mitochondrial membrane potential independent of cytosolic calcium fluxes in mouse cortical neurons.
Pallo SP, Johnson GV. Pallo SP, et al. Neurosci Lett. 2015 Jun 15;597:32-7. doi: 10.1016/j.neulet.2015.04.021. Epub 2015 Apr 15. Neurosci Lett. 2015. PMID: 25888814 Free PMC article. - Synaptic Mitochondria: An Early Target of Amyloid-β and Tau in Alzheimer's Disease.
Torres AK, Jara C, Park-Kang HS, Polanco CM, Tapia D, Alarcón F, de la Peña A, Llanquinao J, Vargas-Mardones G, Indo JA, Inestrosa NC, Tapia-Rojas C. Torres AK, et al. J Alzheimers Dis. 2021;84(4):1391-1414. doi: 10.3233/JAD-215139. J Alzheimers Dis. 2021. PMID: 34719499 Review.
Cited by
- DL0410, a novel dual cholinesterase inhibitor, protects mouse brains against Aβ-induced neuronal damage via the Akt/JNK signaling pathway.
Zhou D, Zhou W, Song JK, Feng ZY, Yang RY, Wu S, Wang L, Liu AL, Du GH. Zhou D, et al. Acta Pharmacol Sin. 2016 Nov;37(11):1401-1412. doi: 10.1038/aps.2016.87. Epub 2016 Aug 8. Acta Pharmacol Sin. 2016. PMID: 27498773 Free PMC article. - VDAC1: A Key Player in the Mitochondrial Landscape of Neurodegeneration.
Argueti-Ostrovsky S, Barel S, Kahn J, Israelson A. Argueti-Ostrovsky S, et al. Biomolecules. 2024 Dec 30;15(1):33. doi: 10.3390/biom15010033. Biomolecules. 2024. PMID: 39858428 Free PMC article. Review. - In vivo functions of Drp1: lessons learned from yeast genetics and mouse knockouts.
Sesaki H, Adachi Y, Kageyama Y, Itoh K, Iijima M. Sesaki H, et al. Biochim Biophys Acta. 2014 Aug;1842(8):1179-85. doi: 10.1016/j.bbadis.2013.11.024. Epub 2013 Dec 8. Biochim Biophys Acta. 2014. PMID: 24326103 Free PMC article. Review. - Pathological Impact of Tau Proteolytical Process on Neuronal and Mitochondrial Function: a Crucial Role in Alzheimer's Disease.
Olesen MA, Quintanilla RA. Olesen MA, et al. Mol Neurobiol. 2023 Oct;60(10):5691-5707. doi: 10.1007/s12035-023-03434-4. Epub 2023 Jun 19. Mol Neurobiol. 2023. PMID: 37332018 Review. - Role of Tau Protein Hyperphosphorylation in Diabetic Retinal Neurodegeneration.
Mu J, Zhang Z, Jiang C, Geng H, Duan J. Mu J, et al. J Ophthalmol. 2025 Mar 12;2025:3278794. doi: 10.1155/joph/3278794. eCollection 2025. J Ophthalmol. 2025. PMID: 40109357 Free PMC article. Review.
References
- Bongarzone ER, Foster L, Bryravan S, Casaccia-Bonnefil P, Schonmann V, Campagnoni AT. Two neuronal cell lines expressing the myelin basic protein gene display differences in their in vitro survival and in their response to glia. J. Neurosci Res. 1998;54:309–319. - PubMed
- Blass JP, Gibson GE, Hoyer S. The role of the metabolic lesion in Alzheimer's disease. J Alzheimer Dis. 2002;4:225–232. - PubMed
- Chung CW, Song YH, Kim IK, Yoon WJ, Ryu BR, Jo DG, Woo HN, Kwon YK, Kim HH, Gwang BJ, Mook-Jung IH, Jung YK. Proapoptotic effects of tau cleavage product generated by caspase-3. Neurobiol Dis. 2001;8:162–172. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical