Binding affinity and specificity of neuromyelitis optica autoantibodies to aquaporin-4 M1/M23 isoforms and orthogonal arrays - PubMed (original) (raw)
Binding affinity and specificity of neuromyelitis optica autoantibodies to aquaporin-4 M1/M23 isoforms and orthogonal arrays
Jonathan M Crane et al. J Biol Chem. 2011.
Abstract
Autoantibodies against astrocyte water channel aquaporin-4 (AQP4) are highly specific for the neuroinflammatory disease neuromyelitis optica (NMO). We measured the binding of NMO autoantibodies to AQP4 in human astrocyte-derived U87MG cells expressing M1 and/or M23 AQP4, or M23 mutants that do not form orthogonal array of particles (OAPs). Binding affinity was quantified by two-color fluorescence ratio imaging of cells stained with NMO serum or a recombinant monoclonal NMO autoantibody (NMO-rAb), together with a C terminus anti-AQP4 antibody. NMO-rAb titrations showed binding with dissociation constants down to 44 ± 7 nm. Different NMO-rAbs and NMO patient sera showed a wide variation in NMO-IgG binding to M1 versus M23 AQP4. Differences in binding affinity rather than stoichiometry accounted for M1 versus M23 binding specificity, with consistently greater affinity of NMO-IgG binding to M23 than M1 AQP4. Binding and OAP measurements in cells expressing different M1:M23 ratios or AQP4 mutants indicated that the differential binding of NMO-IgG to M1 versus M23 was due to OAP assembly rather than to differences in the M1 versus M23 N termini. Purified Fab fragments of NMO-IgG showed similar patterns of AQP4 isoform binding, indicating that structural changes in the AQP4 epitope upon array assembly, and not bivalent cross-linking of whole IgG, result in the greater binding affinity to OAPs. Our study establishes a quantitative assay of NMO-IgG binding to AQP4 and indicates remarkable, OAP-dependent heterogeneity in NMO autoantibody binding specificity.
Figures
FIGURE 1.
Schematic of the two-color ratio imaging method for quantitative measurement of NMO-IgG binding to AQP4 isoforms. A, AQP4 monomers (cylinders) are shown as assembling into tetramers (top) or OAPs (bottom). NMO-IgG (green) binds AQP4 at an extracellular domain, and a reference AQP4 antibody (red) binds on the cytoplasmic side. B, reference AQP4 antibody binds to the C terminus of AQP4, independent of the AQP4 N-terminal isoform and OAP formation.
FIGURE 2.
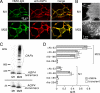
Characterization of stably transfected, AQP4-expressing U87MG cells. A, confocal fluorescence images show U87MG cells stably expressing M1 (top) or M23 (bottom) and labeled with NMO-IgG (green) and C-terminal anti-AQP4 antibody (red). B, TIRF images show distinct OAPs in M23-expressing cells (bottom) and a smooth fluorescence staining pattern in M1-expressing cells (top). C, AQP4 immunoblot following Blue-Native-PAGE (top) and Tricine SDS-PAGE (bottom) of stable AQP4-expressing U87MG cell lysates is shown. D, measured G/R fluorescence ratios in U87MG cells after stable (gray) or transient (white) transfection with M1 or M23 AQP4 and labeled with the indicated recombinant monoclonal NMO-IgG (mean ± S.E. (error bars), n = 4) are shown.
FIGURE 3.
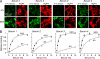
Differential binding of NMO-IgG in NMO patient serum to M1 versus M23 AQP4. A, M1- and M23-expressing U87MG cells stained with 5% NMO serum (green) from four patients and with reference AQP4 antibody (red). B, binding curves for the NMO patient sera to M1 versus M23 AQP4 (mean ± S.E. (error bars), n = 5). Curves represent fits to single-site binding model.
FIGURE 4.
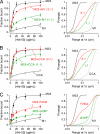
Differential binding of purified monoclonal NMO-IgGs to M1 versus M23 AQP4. A, representative fluorescence micrographs for binding of rAb-53 and rAb-58 (green) as a function of concentration, together with reference AQP4 antibody (red). B, binding curves for rAb-53 (left), rAb-58 (middle), and rAb-186 (right) to M1 versus M23 AQP4 (mean ± S.E. (error bars), n = 5). Curves represent fits to a single-site binding model, with fitted KD = 44 ± 7 n
m
and 2.8 ± 1 μ
m
(rAb-53), 68 ± 9 n
m
and 147 ± 20 n
m
(rAb-58), and 146 ± 25 n
m
and 8.1 ± 3 μ
m
(rAb-186), each for M23 and M1, respectively.
FIGURE 5.
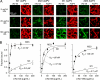
Binding of NMO-IgG to mixtures of M1 and M23 AQP4 and to M23 mutants containing OAP-disrupting mutations. A, binding of rAb-53 (left) and cumulative distributions of diffusion range (right), measured by Qdot single-particle tracking, for M1 and M23 AQP4 mixtures at the indicated ratios (mean ± S.E. (error bars), n = 5). B, binding of rAb-53 (left) and diffusion range (right) for M23 AQP4 with M1 mutant CCA at the indicated ratios (mean ± S.E., n = 5). C, binding of rAb-53 (left) and diffusion range (right) for AQP4 mutants M23-F26Q (red) and M23-G28P (green) (mean ± S.E., n = 5).
FIGURE 6.
Mechanism of increased NMO-IgG binding affinity to OAP-assembled AQP4. A, human IgG (Protein Data Bank ID code 1IGY) (38) and AQP4 (Protein Data Bank ID code 3GD8) (39) crystal structures showing relative size of the AQP4 tetramer compared with spacing between Fab binding sites in whole IgG. B, predictions of bivalent versus monovalent binding mechanisms. AQP4 monomers (cylinders) are shown as assembled in tetramers (M1) or OAPs (M23). NMO-IgG (green) binds either monovalently or bivalently to (unknown) extracellular domains on AQP4. C, binding of monoclonal mouse anti-Myc to cells expressing Myc-tagged M1 versus M23 AQP4. D, relative M1-to-M23 binding of whole IgG or purified Fab fragments of mouse anti-Myc (left), rAb-53 (middle), and rAb-58 (right) at a fixed concentration (mean ± S.E. (error bars), n = 5).
Similar articles
- Neuromyelitis optica IgG does not alter aquaporin-4 water permeability, plasma membrane M1/M23 isoform content, or supramolecular assembly.
Rossi A, Ratelade J, Papadopoulos MC, Bennett JL, Verkman AS. Rossi A, et al. Glia. 2012 Dec;60(12):2027-39. doi: 10.1002/glia.22417. Epub 2012 Sep 14. Glia. 2012. PMID: 22987455 Free PMC article. - Aquaporin-4 orthogonal arrays of particles are the target for neuromyelitis optica autoantibodies.
Nicchia GP, Mastrototaro M, Rossi A, Pisani F, Tortorella C, Ruggieri M, Lia A, Trojano M, Frigeri A, Svelto M. Nicchia GP, et al. Glia. 2009 Oct;57(13):1363-73. doi: 10.1002/glia.20855. Glia. 2009. PMID: 19229993 - Aquaporin-4 autoantibodies in Neuromyelitis Optica: AQP4 isoform-dependent sensitivity and specificity.
Pisani F, Sparaneo A, Tortorella C, Ruggieri M, Trojano M, Mola MG, Nicchia GP, Frigeri A, Svelto M. Pisani F, et al. PLoS One. 2013 Nov 15;8(11):e79185. doi: 10.1371/journal.pone.0079185. eCollection 2013. PLoS One. 2013. PMID: 24260168 Free PMC article. - Aquaporin-4: orthogonal array assembly, CNS functions, and role in neuromyelitis optica.
Verkman AS, Ratelade J, Rossi A, Zhang H, Tradtrantip L. Verkman AS, et al. Acta Pharmacol Sin. 2011 Jun;32(6):702-10. doi: 10.1038/aps.2011.27. Epub 2011 May 9. Acta Pharmacol Sin. 2011. PMID: 21552296 Free PMC article. Review. - Biology of AQP4 and anti-AQP4 antibody: therapeutic implications for NMO.
Verkman AS, Phuan PW, Asavapanumas N, Tradtrantip L. Verkman AS, et al. Brain Pathol. 2013 Nov;23(6):684-95. doi: 10.1111/bpa.12085. Brain Pathol. 2013. PMID: 24118484 Free PMC article. Review.
Cited by
- The cerebrospinal fluid immunoglobulin transcriptome and proteome in neuromyelitis optica reveals central nervous system-specific B cell populations.
Kowarik MC, Dzieciatkowska M, Wemlinger S, Ritchie AM, Hemmer B, Owens GP, Bennett JL. Kowarik MC, et al. J Neuroinflammation. 2015 Jan 28;12:19. doi: 10.1186/s12974-015-0240-9. J Neuroinflammation. 2015. PMID: 25626447 Free PMC article. - Discovery of peptoid ligands for anti-aquaporin 4 antibodies.
Raveendra BL, Wu H, Baccala R, Reddy MM, Schilke J, Bennett JL, Theofilopoulos AN, Kodadek T. Raveendra BL, et al. Chem Biol. 2013 Mar 21;20(3):351-9. doi: 10.1016/j.chembiol.2012.12.009. Chem Biol. 2013. PMID: 23521793 Free PMC article. - Accuracy of the Fluorescence-Activated Cell Sorting Assay for the Aquaporin-4 Antibody (AQP4-Ab): Comparison with the Commercial AQP4-Ab Assay Kit.
Yang J, Kim SM, Kim YJ, Cheon SY, Kim B, Jung KC, Park KS. Yang J, et al. PLoS One. 2016 Sep 22;11(9):e0162900. doi: 10.1371/journal.pone.0162900. eCollection 2016. PLoS One. 2016. PMID: 27658059 Free PMC article. - Aquaporin-4 antibody isoform binding specificities do not explain clinical variations in NMO.
Kitley J, Woodhall M, Leite MI, Palace J, Vincent A, Waters P. Kitley J, et al. Neurol Neuroimmunol Neuroinflamm. 2015 Jun 18;2(4):e121. doi: 10.1212/NXI.0000000000000121. eCollection 2015 Aug. Neurol Neuroimmunol Neuroinflamm. 2015. PMID: 26140280 Free PMC article. - Relationship Between Neuromyelitis Optica Spectrum Disorder and Sjögren's Syndrome: Central Nervous System Extraglandular Disease or Unrelated, Co-Occurring Autoimmunity?
Birnbaum J, Atri NM, Baer AN, Cimbro R, Montagne J, Casciola-Rosen L. Birnbaum J, et al. Arthritis Care Res (Hoboken). 2017 Jul;69(7):1069-1075. doi: 10.1002/acr.23107. Arthritis Care Res (Hoboken). 2017. PMID: 27696784 Free PMC article.
References
- Matiello M., Lennon V. A., Jacob A., Pittock S. J., Lucchinetti C. F., Wingerchuk D. M., Weinshenker B. G. (2008) Neurology 70, 2197–2200 - PubMed
- Bradl M., Misu T., Takahashi T., Watanabe M., Mader S., Reindl M., Adzemovic M., Bauer J., Berger T., Fujihara K., Itoyama Y., Lassmann H. (2009) Ann. Neurol. 66, 630–643 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL73856/HL/NHLBI NIH HHS/United States
- R01 EY013574/EY/NEI NIH HHS/United States
- DK86125/DK/NIDDK NIH HHS/United States
- R01 EB000415/EB/NIBIB NIH HHS/United States
- R01 DK035124/DK/NIDDK NIH HHS/United States
- DK72517/DK/NIDDK NIH HHS/United States
- EY13574/EY/NEI NIH HHS/United States
- DK35124/DK/NIDDK NIH HHS/United States
- R01 HL073856/HL/NHLBI NIH HHS/United States
- P30 DK072517/DK/NIDDK NIH HHS/United States
- RC1 DK086125/DK/NIDDK NIH HHS/United States
- EB00415/EB/NIBIB NIH HHS/United States
- R37 DK035124/DK/NIDDK NIH HHS/United States
- R37 EB000415/EB/NIBIB NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources