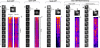A conserved coatomer-related complex containing Sec13 and Seh1 dynamically associates with the vacuole in Saccharomyces cerevisiae - PubMed (original) (raw)
. 2011 Jun;10(6):M110.006478.
doi: 10.1074/mcp.M110.006478. Epub 2011 Mar 31.
Francois Waharte, Avner Schlessinger, Ursula Pieper, Damien P Devos, Ileana M Cristea, Rosemary Williams, Jean Salamero, Brian T Chait, Andrej Sali, Mark C Field, Michael P Rout, Catherine Dargemont
Affiliations
- PMID: 21454883
- PMCID: PMC3108837
- DOI: 10.1074/mcp.M110.006478
A conserved coatomer-related complex containing Sec13 and Seh1 dynamically associates with the vacuole in Saccharomyces cerevisiae
Svetlana Dokudovskaya et al. Mol Cell Proteomics. 2011 Jun.
Abstract
The presence of multiple membrane-bound intracellular compartments is a major feature of eukaryotic cells. Many of the proteins required for formation and maintenance of these compartments share an evolutionary history. Here, we identify the SEA (Seh1-associated) protein complex in yeast that contains the nucleoporin Seh1 and Sec13, the latter subunit of both the nuclear pore complex and the COPII coating complex. The SEA complex also contains Npr2 and Npr3 proteins (upstream regulators of TORC1 kinase) and four previously uncharacterized proteins (Sea1-Sea4). Combined computational and biochemical approaches indicate that the SEA complex proteins possess structural characteristics similar to the membrane coating complexes COPI, COPII, the nuclear pore complex, and, in particular, the related Vps class C vesicle tethering complexes HOPS and CORVET. The SEA complex dynamically associates with the vacuole in vivo. Genetic assays indicate a role for the SEA complex in intracellular trafficking, amino acid biogenesis, and response to nitrogen starvation. These data demonstrate that the SEA complex is an additional member of a family of membrane coating and vesicle tethering assemblies, extending the repertoire of protocoatomer-related complexes.
Figures
Fig. 1.
Identification of the Seh1 associated complex. Immunoprecipitation of Protein A-tagged proteins (indicated in blue) was performed as described under “Experimental Procedures.” SEA complex proteins and their partners were resolved by SDS-PAGE and visualized by Coomassie blue. Proteins identified by mass spectrometry (
supplemental Table S2
) are listed to the right of the gel lanes (IgG contaminant is indicated in gray). Molecular weight markers are indicated to the left of the panel. Each individual gel image was differentially scaled along its length so that its molecular mass standards aligned to a single reference set of molecular mass standards. Contrast was adjusted to improve visibility. All original gel figures are available upon request.
Fig. 2.
Secondary structure prediction and fold assessment of yeast S. cerevisiae and human SEA complex proteins. Secondary structure predictions for each residue by PSIPRED are shown as vertical lines with α-helices colored in magenta and β-strands in cyan. The length of the column is proportional to the confidence of the secondary structure prediction (24). Disordered regions were predicted using DISOPRED2 (yellow) or IUPRED (green). Assigned folds (
supplemental Table S3
) are also shown, visualized with ribbon diagrams of representative atomic structures from Protein Data Bank (
).
Fig. 3.
SEA complex proteins have evolutionarily conserved structural characteristics similar to membrane coats. A, Protease accessibility laddering (PAL) analysis of SEA complex proteins. PAL readily detects domain boundaries and flexible loops within proteins (23). Protein A-tagged SEA proteins were purified on magnetic beads in their natively folded state. While attached to the beads, proteins were probed with proteases (Asp-D, Lys-C and Trypsin). Proteolytic fragments, containing C-terminal PrA tag were eluted and detected by immunoblotting with IgG-HRP. Shown are immunoblots of PAL digests for PrA-tagged versions of Sea1, Sea2, Sea3, and Sea4. Full-length proteins are indicated with a dot and proteolytic fragments with a star and a letter. Sites of proteolysis are marked with arrows on a secondary structure prediction map (shown to the right of each gel). Uncertainties in the precise cleavage positions are indicated by lines to the left of the map (see also
supplemental Table S4
). B, Sea4 forms a dimer with Seh1 similar to the COPII coat complex Sec31-Sec13. Note, that in this experiment Sea4-PrA was expressed in the cells deleted for Sea3 and immunoprecipitated under stringent conditions with 1
m
NaCl present both in the extraction and washing buffers (see Experimental procedures). Therefore the resulting complex is different than the one shown on Fig. 1, lane #5. Sec31-PrA expressed in wild type yeast (lane #2) was immunoprecipitated as described under “Experimental Procedures.” Eluted proteins were resolved on SDS-PAGE gels, stained with Coomassie blue and identified by mass spectrometry (
supplemental Table S2
). Arrows indicate predicted folds. Seh1 and Sec13 are indicated as 6-blade β-propeller, according to their x-ray structures (15, 76).
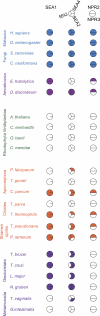
Fig. 4.
Distribution of the SEA complex proteins across the eukaryota. Representative genomes were searched as described under “Experimental Procedures” and shown as a Coulson plot. Filled sectors represent evidence for orthologues, while open sectors indicate that no orthologue found. Individual taxa are color coded as follows: Opistokhonta, blue; Amoebozoa, light purple; Planta, green; Chromalveolata, orange; Excavata, dark purple. Lower order groupings are indicated, and a key to the factors is given at top. Factors are subdivided into three groups: Sea1, Sea2–4, Npr2, and Npr3. Accession numbers and additional data are listed in the
supplemental Table S5
.
Fig. 5.
Proteins of the SEA complex are localized around vacuole membrane. Live florescence images of the SEA complex proteins genomically expressing GFP at their C terminus. A, Principle of the Sum Intensity Projection (SIP) algorithm, applied for localizing Seh1-GFP. Living cells were analyzed using a confocal-spinning disk microscope with low illumination power. Intensity values on a given pixel of the image are summed over all images in the time sequence to give the final image (right). B, Yeast cells were visualized by Nomarski optics (“DIC” row). GFP signals shown in the “GFP Sum” row were obtained by SIP or Maximum Intensity Projections of image sequences (duration or number of frames) taken with high exposure times (∼500 ms) to increase signal-to-noise ratio. Scale bar = 5 μm. C, Characterization of association of SEA complex proteins with enriched vacuole fraction. Total vacuole fractions (T) prepared from indicated PrA-tagged SEA complex proteins were treated with 0.1
m
Na2CO3 prior to centrifugation at 100,000 × g. The resulting supernatant (S) and pellet (P) were analyzed by Western blotting with an IgG-HRP antibody. The distribution of vacuole integral membrane protein Vph1 was visualized with an anti-Vph1 antibody.
Fig. 6.
Dynamics of the SEA complex proteins. (A, D, G, J, M): image sequence showing the dynamics of the fluorescently tagged proteins (time interval between two images is 1.4 s for Seh1 and 1.0 s for all other proteins). The dynamics of Sea1-GFP shown at (J) corresponds to the
Movie S1
. (B, E, H, K, N): SIP images showing the average localization of the protein with white lines indicating the regions used for generation of kymograms. Seh1-GFP localization at the nuclear envelope and at the vacuole membrane is indicated with “N” and “V”, respectively. (C, F, I, L, O) Kymogram representations of the image sequences along horizontal (x) or vertical (t) lines as shown on SIP images. Intensity traces appear more blurry on the Sea4-mCherry kymogram, because of a difference in optical resolution due the red shift of the fluorescence emission spectrum of the mCherry tag compared with the GFP tag, as well as a difference in the incidence angle of the 561 nm laser used for detection of mCherry compared with the 491 nm laser (GFP detection). Scale bar = 2 μm.
Fig. 7.
SEA complex proteins are enriched in the fraction of the small compartments and are not integral to the membrane. A, Distribution of SEA complex proteins and membrane components of various organelles between different fractions generated by subcellular fractionation. The yeast cell lysates prepared from strains, containing indicated PrA-tagged SEA complex proteins were subjected to a low-force centrifugation to pellet unlysed cells and large aggregates. The cleared lysate (S5) was further subjected to sequential centrifugation steps to generate a 13,000 × g pellet (P13) and supernatant (S13), a 100,000 × g pellet (P100), and a 100,000 × g supernatant (S100). The P13 fraction contains plasma membrane and membranes of big organelles (e.g. nuclear, vacuolar, mitochondrial, and ER); P100 fraction is enriched in smaller compartments (Golgi complex, transport vesicles, and ribosomes); S100 fraction contains soluble cytoplasmic proteins and released peripheral membrane proteins. Samples of fractions were normalized to cell equivalents by differential loading on SDS-PAGE, which was further subjected to Western blotting and probed either with IgG-HRP to reveal PrA-tagged SEA complex proteins or with appropriate antibodies against control proteins (indicated to the left of the blot). Integral membrane proteins of the vacuole (Vph1), mitochondria (Por1), and ER (Dpm1) were precipitated in the P13 fraction. The vacuolar peripheral membrane protein Vma2 is equally distributed between P13 and S100. Vps10, which cycles between the late-Golgi and prevacuolar endosome-like compartments, and COPII member Sec23 are found in P13 and P100. B, S13 fractions were sedimented on a 5–20% sucrose gradient. Fractions were collected and analyzed by immunoblotting of PrA tag. Immunoblot from a typical analysis indicating a distribution between fractions of Sea3-PrA. C, A graph showing the sedimentation profile of six SEA complex proteins. The proteins are distributed in two sub-populations.
Fig. 8.
A survey of phenotypes in the SEA complex deletion strains. A, Sensitivity to 50 n
m
rapamycin of single and double deletion strains of sea2-sea4. Indicated deletion strains were spotted in 5-fold dilution steps on YPD plates complemented with 50 n
m
rapamycin and grown for 4 days at 30 °C. B, Survival of sea2-sea4 double deletion strains after 7 days of nitrogen starvation. C, Wild type and indicated deletion strains transformed with a plasmid containing GFP-ATG8 were grown as described under “Experimental Procedures” and examined under a fluorescent microscope. Scale bar = 5 μm. D, Strains were grown as described under “Experimental Procedures”. Samples were taken at indicated time points and analyzed by Western blotting with anti-GFP antibody.
Similar articles
- A novel coatomer-related SEA complex dynamically associates with the vacuole in yeast and is implicated in the response to nitrogen starvation.
Dokudovskaya S, Rout MP. Dokudovskaya S, et al. Autophagy. 2011 Nov;7(11):1392-3. doi: 10.4161/auto.7.11.17347. Epub 2011 Nov 1. Autophagy. 2011. PMID: 21804352 Free PMC article. - SEACing the GAP that nEGOCiates TORC1 activation: evolutionary conservation of Rag GTPase regulation.
Panchaud N, Péli-Gulli MP, De Virgilio C. Panchaud N, et al. Cell Cycle. 2013 Sep 15;12(18):2948-52. doi: 10.4161/cc.26000. Epub 2013 Aug 13. Cell Cycle. 2013. PMID: 23974112 Free PMC article. - The CORVET tethering complex interacts with the yeast Rab5 homolog Vps21 and is involved in endo-lysosomal biogenesis.
Peplowska K, Markgraf DF, Ostrowicz CW, Bange G, Ungermann C. Peplowska K, et al. Dev Cell. 2007 May;12(5):739-50. doi: 10.1016/j.devcel.2007.03.006. Dev Cell. 2007. PMID: 17488625 - Making COPII coats.
Kirchhausen T. Kirchhausen T. Cell. 2007 Jun 29;129(7):1251-2. doi: 10.1016/j.cell.2007.06.015. Cell. 2007. PMID: 17604713 Review. - Membrane-coating lattice scaffolds in the nuclear pore and vesicle coats: commonalities, differences, challenges.
Leksa NC, Schwartz TU. Leksa NC, et al. Nucleus. 2010 Jul-Aug;1(4):314-8. doi: 10.4161/nucl.1.4.11798. Epub 2010 Mar 12. Nucleus. 2010. PMID: 21327078 Free PMC article. Review.
Cited by
- Cryo-EM structure of the SEA complex.
Tafur L, Hinterndorfer K, Gabus C, Lamanna C, Bergmann A, Sadian Y, Hamdi F, Kyrilis FL, Kastritis PL, Loewith R. Tafur L, et al. Nature. 2022 Nov;611(7935):399-404. doi: 10.1038/s41586-022-05370-0. Epub 2022 Oct 26. Nature. 2022. PMID: 36289347 Free PMC article. - A jumbo problem: mapping the structure and functions of the nuclear pore complex.
Fernandez-Martinez J, Rout MP. Fernandez-Martinez J, et al. Curr Opin Cell Biol. 2012 Feb;24(1):92-9. doi: 10.1016/j.ceb.2011.12.013. Epub 2012 Feb 8. Curr Opin Cell Biol. 2012. PMID: 22321828 Free PMC article. Review. - The lysosome as a command-and-control center for cellular metabolism.
Lim CY, Zoncu R. Lim CY, et al. J Cell Biol. 2016 Sep 12;214(6):653-64. doi: 10.1083/jcb.201607005. J Cell Biol. 2016. PMID: 27621362 Free PMC article. Review. - The Structure of the Nuclear Pore Complex (An Update).
Lin DH, Hoelz A. Lin DH, et al. Annu Rev Biochem. 2019 Jun 20;88:725-783. doi: 10.1146/annurev-biochem-062917-011901. Epub 2019 Mar 18. Annu Rev Biochem. 2019. PMID: 30883195 Free PMC article. Review. - Seh1 targets GATOR2 and Nup153 to mitotic chromosomes.
Platani M, Samejima I, Samejima K, Kanemaki MT, Earnshaw WC. Platani M, et al. J Cell Sci. 2018 May 1;131(9):jcs213140. doi: 10.1242/jcs.213140. J Cell Sci. 2018. PMID: 29618633 Free PMC article.
References
- Dacks J. B., Field M. C. (2007) Evolution of the eukaryotic membrane-trafficking system: origin, tempo and mode. J. Cell Sci. 120, 2977–2985 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 090007/WT_/Wellcome Trust/United Kingdom
- R01 GM083960/GM/NIGMS NIH HHS/United States
- P41 RR000862/RR/NCRR NIH HHS/United States
- U54 RR022220/RR/NCRR NIH HHS/United States
- F32 GM088991/GM/NIGMS NIH HHS/United States
- R01 GM054762/GM/NIGMS NIH HHS/United States
- DP1 DA026192-04/DA/NIDA NIH HHS/United States
- RR00862/RR/NCRR NIH HHS/United States
- R01 GM62427/GM/NIGMS NIH HHS/United States
- R01 GM062427/GM/NIGMS NIH HHS/United States
- DP1 DA026192/DA/NIDA NIH HHS/United States
- F32 GM088991-01A1/GM/NIGMS NIH HHS/United States
- R01 GM54762/GM/NIGMS NIH HHS/United States
- DP1DA026192/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous