AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice - PubMed (original) (raw)
. 2011 Apr 6;13(4):376-388.
doi: 10.1016/j.cmet.2011.03.009.
Shanqin Xu 1, Maria M Mihaylova 2, Bin Zheng 3, Xiuyun Hou 1, Bingbing Jiang 1, Ogyi Park 4, Zhijun Luo 1, Etienne Lefai 5, John Y-J Shyy 6, Bin Gao 4, Michel Wierzbicki 7, Tony J Verbeuren 7, Reuben J Shaw 2, Richard A Cohen 1, Mengwei Zang 8
Affiliations
- PMID: 21459323
- PMCID: PMC3086578
- DOI: 10.1016/j.cmet.2011.03.009
AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice
Yu Li et al. Cell Metab. 2011.
Abstract
AMPK has emerged as a critical mechanism for salutary effects of polyphenols on lipid metabolic disorders in type 1 and type 2 diabetes. Here we demonstrate that AMPK interacts with and directly phosphorylates sterol regulatory element binding proteins (SREBP-1c and -2). Ser372 phosphorylation of SREBP-1c by AMPK is necessary for inhibition of proteolytic processing and transcriptional activity of SREBP-1c in response to polyphenols and metformin. AMPK stimulates Ser372 phosphorylation, suppresses SREBP-1c cleavage and nuclear translocation, and represses SREBP-1c target gene expression in hepatocytes exposed to high glucose, leading to reduced lipogenesis and lipid accumulation. Hepatic activation of AMPK by the synthetic polyphenol S17834 protects against hepatic steatosis, hyperlipidemia, and accelerated atherosclerosis in diet-induced insulin-resistant LDL receptor-deficient mice in part through phosphorylation of SREBP-1c Ser372 and suppression of SREBP-1c- and -2-dependent lipogenesis. AMPK-dependent phosphorylation of SREBP may offer therapeutic strategies to combat insulin resistance, dyslipidemia, and atherosclerosis.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
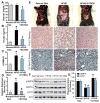
Fig. 1. A synthetic polyphenol, S17834, stimulates AMPK activity and protects against insulin resistance and hepatic steatosis in high fat, high sucrose (HFHS) diet-fed LDL receptor-deficient (LDLR−/−) mice
A–C. Administration of S17834 for 16 weeks effectively improves insulin resistance in mice fed the type 2 diabetogenic diet (HFHS diet). Plasma insulin levels, blood glucose, and calculated HOMA-IR were assessed in mice fed a normal chow diet (n=10–15), a HFHS diet (n=30), and a HFHS diet supplemented with S17834 (HFHS+S17834, n=30). D and E. Representative gross morphology of the mouse livers, H & E staining and Oil Red O staining of liver sections. F. AMPK activity is suppressed by HFHS diet and restored by S17834 in the liver of insulin resistant mice. G. Densitometric quantification of the phosphorylation of AMPK and ACC. The data are presented as the mean S.E.M., n= 8. *P<0.05, vs normal diet mice; #P<0.05, vs HFHS-fed mice.
Fig. 2. AMPK activation by S17834 attenuates the proteolytic processing of SREBP-1 and SREBP-2, inhibits expression of their target lipogenic enzymes, and reduces lipid accumulation in the liver of the insulin resistant LDLR−/− mice
A. The mature, active nuclear form of hepatic SREBP-1 is increased in HFHS-fed mice, and the increase is completely blocked by S17834 treatment. P and N denote the precursor (~125 kDa) and cleaved nuclear (~68 kDa) forms of SREBP-1. B. Enhanced SREBP-2 processing by HFHS diet is reduced in the liver of S17834-treated mice. C. Densitometric quantification of cleaved forms of hepatic SREBP-1 and −2. D and E. The transcription of genes involved in triglyceride and cholesterol biosynthesis is decreased in the liver of S17834-treated mice. The mRNA amounts of genes encoding SREBP-1a, SREBP-1c, and SREBP-2 (D), as well as genes encoding ACC1, FAS, SCD1, HMGCS, and HMGCR (E) in the mouse livers were determined by real-time RT-PCR. F–H. S17834 decreases the protein expression of lipogenic enzymes including FAS and HMGCR in the livers of HFHS diet-fed mice. The strong staining for FAS and HMGCR were primarily located in hepatocytes around the central and peripheral veins of the liver of HFHS diet-fed mice. The horizontal bars represent average staining intensity. I. S17834 treatment inhibits lipid accumulation in the liver of HFHS-fed mice. The data are presented as the mean S.E.M., n=7–8. *P<0.05, vs normal diet mice; #P<0.05, vs HFHS-fed mice.
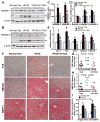
Fig. 3. AMPK activation by S17834 inhibits accelerated aortic atherosclerosis and vascular inflammation in insulin resistant LDLR−/− mice by preventing dyslipidemia
A. and B. Time-course changes of plasma triglyceride and total cholesterol levels in mice following a 16 h fast are presented as the mean S.E.M, n=8–16. C. Lipoprotein distribution in LDLR−/− mice after 16 weeks of normal diet (green), HFHS diet (red), and HFHS diet supplemented with S17834 (blue). Pooled plasma samples for lipoprotein distribution were determined by FPLC, followed by cholesterol analysis of each fraction. Data are represented as an average (n=3–6) distribution of total cholesterol. D. The increased plasma VLDL/LDL cholesterol in HFHS-fed LDLR−/− mice is attenuated by S17384. Quantification of plasma VLDL/LDL cholesterol (VLDL/LDL-C) and HDL cholesterol (HDL-C) is shown. E. Quantification of atherosclerotic lesion areas in cross-sections of the proximal aorta was performed by computer-assisted image analysis. Total lesion area per section in the entire aortic root was determined and presented as the mean ± S.E.M., n = 8, *P<0.05, vs normal diet mice; #P<0.05, vs HFHS-fed mice. F. Linear regression analysis between plasma total cholesterol levels and aortic atherosclerotic lesions in insulin resistant LDLR−/− mice. Each point represents an individual value of one mouse. G. Representative Oil Red O staining of cross-sections of aortic root in the heart of LDLR−/− mice. H. Expression of vascular cell adhesion molecule-1 (VCAM-1) is reduced in the aorta of S17834-treated insulin resistant mice, as determined by immunohistochemical staining. I. Semi-quantification analysis by ImageJ software of staining intensity of VCAM-1 in atherosclerotic plaques of ascending aortic arch of LDLR−/− mice is shown. The bar graph represents the results from 3 separate mice in each group.
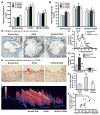
Fig. 4. AMPK suppresses the cleavage processing and nuclear translocation of SREBP-1 in human HepG2 cells or in diabetic mouse livers
A. and B. Overexpression of DN-AMPK abolishes the inhibitory effect of resveratrol on accumulation of nuclear SREBP-1 in HepG2 cells exposed to high glucose or high glucose plus insulin. C. DN-AMPK abrogates the effect of S17834 to reduce the nuclear SREBP-1 in isolated hepatocytes. After a 24-h period of infection with Ad-GFP or Ad-DN-AMPK, HepG2 cells or primary hepatocytes were incubated in serum free DMEM containing 5.5 mM overnight and treated for an additional 24 h with resveratrol or S17834 in the presence of high glucose (30 mM) or high glucose (30 mM) and insulin (100 nM). D. Enhanced nuclear translocation of SREBP-1 in response to high glucose or high glucose plus insulin is prevented by either S17834 or metformin in HepG2 cells. Immunoblot analysis of SREBP-1 in cytoplasmic and nuclear extracts is shown. E. Confocal of immunofluorescent images show SREBP-1 staining (Green) and nuclear staining with propidium iodide (PI, Red) in HepG2 cells. F. S17834 and metformin decrease lipid accumulation in HepG2 cells exposed to high glucose (HG) plus insulin, as reflected by Oil Red O staining. G. Increased nuclear translocation of SREBP-1 is increased in the hepatocytes of insulin resistant mice and eliminated by S17834. A representative confocal microscopy image of immunofluorescent staining of liver sections for SREBP-1 (Green) and nuclear (Red) is shown. Arrows represent SREBP-1 localization in nucleus of hepatocytes, original magnification, 60.
Fig. 5. AMPK represses the transcriptional activity of SREBP-1c and its lipogenic target gene
A. CA-AMPK is sufficient to suppress enhanced SREBP-1-dependent de novo lipogenic gene expression in HepG2 cells exposed to high glucose. The mRNAs encoding SREBP-1a, −1c and FAS were analyzed by real-time RT-PCR. B. AMPK is required for polyphenols to reduce mRNA levels of SREBP-1c and FAS in HepG2 cells exposed to high glucose. Data are presented as the mean ± S.E.M., n=3–4, *P<0.05, vs normal glucose; #P<0.05, vs high glucose. C. SRE motif is responsible for AMPK to repress transcriptional activity on SREBP-1c promoter. The proximal promoter regulatory region of human SREBP-1c contains its cis-acting elements: two SRE elements and the putative NF-Y and SP-1 sites. HepG2 cells were cotransfected with empty plasmid pGL3, luciferase reporter plasmids containing wild type human SREBP-1c promoters (−1470/+90 and −257/+90), or the mutant reporter with disrupted SRE, together with Renilla luciferase reporter plasmid pRL-SV40. Thirty two hours post transfection, cells were cultured in serum-free DMEM and treated with or without polyphenols for 16 h. D. DN-AMPK enhances the transcription activity of SREBP-1c promoter (−1470/+90) and abrogates the inhibitory effect of resveratrol in HepG2 cells. E. AMPK−/− MEFs exhibit enhanced FAS promoter activity. *P<0.05, vs AMPK+/+MEFs. F. Suppression of FAS gene transcription in response to AICAR and S17834 is diminished by DN-SREBP-1c. G. AMPK suppresses FAS promoter activity in a SREBP-1c dependent manner. *P<0.05, vs untreated group. *P<0.05, vs treatment group.
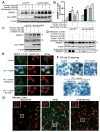
Fig. 6. AMPK catalytic α subunit associates with the precursor and nuclear forms of SREBP-1 or SREBP-2 isoforms
A. AMPKα1 or α2 subunit physically associates with endogenous SREBP-1 precursor in HEK293T cells. B. GST and GST- AMPKα1 were transiently transfected into HEK293T cells and purified with GSH Sepharose beads. The precipitates and lysates were individually immunoblotted with antibodies against SREBP-1 or GST. C. Endogenous AMPKα subunit interacts with nuclear SREBP-1c. GST and GST-nuclear SREBP-1c were transfected into HEK293T cells and purified by GST pull down. D. The active form of AMPK preferentially interacts with endogenous SREBP-1 precursor. HEK293T cells were transfected with FLAG- AMPKα1, wild type, constitutive active (CA), or dominant negative (DN) mutants. E. AMPK activation by resveratrol enhances the association between AMPKα and SREBP-1 precursor. HEK293T cells were transfected with myc-tagged wild type AMPKα1 and treated with resveratrol (10 μM, 16 h). F. AMPK activation by S17834 decreases cleavage processing of overexpressed myc-tagged SREBP-1c precursor in HEK293T cells.
Fig. 7. SREBP-1c is a direct target of AMPK
A. Active AMPK phosphorylates human SREBP-1c at Ser372 in vitro. Purified recombinant GST-tagged nuclear forms of SREBP-1c, wild type (WT), the mutations of S372A or S336A, from transfected HEK293T cells, were incubated with purified rat AMPK in the presence of [32P]-ATP and 100 μM of ATP and AMP at 30°C for 30 min. Phosphorylation of SREBP-1c was visualized by 32P-autoradiography or by immunoblots with phosphor-specific Ser372 antibody in the in vitro kinase assay. B. AMPK activation by phenformin specifically stimulates Ser372 phosphorylation of SREBP-1c. HEK293T cells expressing GST-tagged human full-length SREBP-1c, wild type (WT) or S372A mutant, were treated with phenformin (5 mM) for 1 h. Total cell lysates were Immunoblotted with phospho-specific Ser372 and total SREBP-1 antibodies. C. AMPK is required for Ser372 phosphorylation in response to polyphenols and AICAR. AMPK+/+ or AMPKα1/α2 double knockout (AMPK−/−) MEFs were treated with AMPK activators for 1 h. D. AMPK deficient cells exhibit the inability of AMPK activators to repress autoregulation of nuclear SREBP-1c. AMPK+/+ and AMPK−/− MEFs were co-transfected with the plasmids encoding nuclear SREBP-1c and FAS promoter and treated with AMPK activators for 16 h. E. Ser372 phosphorylation of SREBP-1c is required for the inhibition of cleavage of SREBP-1c in response to S17834 in HEK293T cells. F. The mutation of full-length SREBP-1c S372A enhances the basal transcription of SREBP-1c promoter (−257/+90) and abrogates the suppression of SREBP-1c gene transcription in response to AMPK activators in HepG2 cells. *P<0.05, vs untreated group; **#**P<0.05, vs treatment group. G. DN-AMPK diminishes polyphenol-induced phosphorylation of SREBP-1c in primary mouse hepatocytes under high glucose conditions. H. AMPK activation by S17834 counteracts impaired Ser372 phosphorylation of SREBP-1c precursor in the liver of insulin resistant LDLR−/− mice. I. Proposed model of the phosphorylation regulation of SREBP-1c and −2 by AMPK in the liver: potential therapeutic implication in hepatic steatosis, insulin resistance and risk of atherosclerosis.
Similar articles
- GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice.
Kammoun HL, Chabanon H, Hainault I, Luquet S, Magnan C, Koike T, Ferré P, Foufelle F. Kammoun HL, et al. J Clin Invest. 2009 May;119(5):1201-15. doi: 10.1172/JCI37007. Epub 2009 Apr 13. J Clin Invest. 2009. PMID: 19363290 Free PMC article. - Docosahexaenoic acid inhibits proteolytic processing of sterol regulatory element-binding protein-1c (SREBP-1c) via activation of AMP-activated kinase.
Deng X, Dong Q, Bridges D, Raghow R, Park EA, Elam MB. Deng X, et al. Biochim Biophys Acta. 2015 Dec;1851(12):1521-9. doi: 10.1016/j.bbalip.2015.08.007. Epub 2015 Aug 29. Biochim Biophys Acta. 2015. PMID: 26327595 - Activation of sterol regulatory element binding protein and NLRP3 inflammasome in atherosclerotic lesion development in diabetic pigs.
Li Y, Xu S, Jiang B, Cohen RA, Zang M. Li Y, et al. PLoS One. 2013 Jun 25;8(6):e67532. doi: 10.1371/journal.pone.0067532. Print 2013. PLoS One. 2013. PMID: 23825667 Free PMC article. - Hepatic steatosis: a role for de novo lipogenesis and the transcription factor SREBP-1c.
Ferré P, Foufelle F. Ferré P, et al. Diabetes Obes Metab. 2010 Oct;12 Suppl 2:83-92. doi: 10.1111/j.1463-1326.2010.01275.x. Diabetes Obes Metab. 2010. PMID: 21029304 Review. - SREBP-1c and TFE3, energy transcription factors that regulate hepatic insulin signaling.
Shimano H. Shimano H. J Mol Med (Berl). 2007 May;85(5):437-44. doi: 10.1007/s00109-007-0158-5. Epub 2007 Feb 6. J Mol Med (Berl). 2007. PMID: 17279346 Review.
Cited by
- Sterol regulatory element binding protein 2 activation of NLRP3 inflammasome in endothelium mediates hemodynamic-induced atherosclerosis susceptibility.
Xiao H, Lu M, Lin TY, Chen Z, Chen G, Wang WC, Marin T, Shentu TP, Wen L, Gongol B, Sun W, Liang X, Chen J, Huang HD, Pedra JH, Johnson DA, Shyy JY. Xiao H, et al. Circulation. 2013 Aug 6;128(6):632-42. doi: 10.1161/CIRCULATIONAHA.113.002714. Epub 2013 Jul 9. Circulation. 2013. PMID: 23838163 Free PMC article. - The autophagy protein Becn1 improves insulin sensitivity by promoting adiponectin secretion via exocyst binding.
Kuramoto K, Kim YJ, Hong JH, He C. Kuramoto K, et al. Cell Rep. 2021 May 25;35(8):109184. doi: 10.1016/j.celrep.2021.109184. Cell Rep. 2021. PMID: 34038729 Free PMC article. - Drynaria rhizome water extract alleviates high‑fat diet‑induced obesity in mice.
Gil TY, Park J, Park YJ, Kim HJ, Cominguez DC, An HJ. Gil TY, et al. Mol Med Rep. 2024 Feb;29(2):30. doi: 10.3892/mmr.2023.13153. Epub 2023 Dec 22. Mol Med Rep. 2024. PMID: 38131179 Free PMC article. - Phytochemical components analysis and hypolipidemic effect on hyperlipidemia mice of the aerial parts from Allium sativum.
Hu B, Hu H, Peng D, Wei Z, Wang Q, Kuang H. Hu B, et al. Front Nutr. 2024 Jul 25;11:1422857. doi: 10.3389/fnut.2024.1422857. eCollection 2024. Front Nutr. 2024. PMID: 39119464 Free PMC article. - Carotenoids in orange carrots mitigate non-alcoholic fatty liver disease progression.
Balbuena E, Cheng J, Eroglu A. Balbuena E, et al. Front Nutr. 2022 Sep 26;9:987103. doi: 10.3389/fnut.2022.987103. eCollection 2022. Front Nutr. 2022. PMID: 36225879 Free PMC article.
References
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang MY, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. - PMC - PubMed
- Cayatte AJ, Rupin A, Oliver-Krasinski J, Maitland K, Sansilvestri-Morel P, Boussard MF, Wierzbicki M, Verbeuren TJ, Cohen RA. S17834, a new inhibitor of cell adhesion and atherosclerosis that targets NADPH oxidase. Arteriosclerosis Thrombosis and Vascular Biology. 2001;21:1577–1584. - PubMed
- Costet P, Cariou B, Lambert G, Lalanne F, Lardeux B, Jarnoux AL, Grefhorst A, Staels B, Krempf M. Hepatic PCSK9 expression is regulated by nutritional status via insulin and sterol regulatory element-binding protein 1c. J Biol Chem. 2006;281:6211–6218. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK59637/DK/NIDDK NIH HHS/United States
- P01 HL068758/HL/NHLBI NIH HHS/United States
- R21 AA021181/AA/NIAAA NIH HHS/United States
- R01 DK080425/DK/NIDDK NIH HHS/United States
- R01 DK076942-02/DK/NIDDK NIH HHS/United States
- U24 DK059637/DK/NIDDK NIH HHS/United States
- R01 DK076942/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases