Diagnosis of prostate cancer using differentially expressed genes in stroma - PubMed (original) (raw)
. 2011 Apr 1;71(7):2476-87.
doi: 10.1158/0008-5472.CAN-10-2585.
Yipeng Wang, Anne Sawyers, Huazhen Yao, Farahnaz Rahmatpanah, Xiao-Qin Xia, Qiang Xu, Rebecca Pio, Tolga Turan, James A Koziol, Steve Goodison, Philip Carpenter, Jessica Wang-Rodriguez, Anne Simoneau, Frank Meyskens, Manuel Sutton, Waldemar Lernhardt, Thomas Beach, Joseph Monforte, Michael McClelland, Dan Mercola
Affiliations
- PMID: 21459804
- PMCID: PMC3071046
- DOI: 10.1158/0008-5472.CAN-10-2585
Diagnosis of prostate cancer using differentially expressed genes in stroma
Zhenyu Jia et al. Cancer Res. 2011.
Abstract
More than one million prostate biopsies are performed in the United States every year. A failure to find cancer is not definitive in a significant percentage of patients due to the presence of equivocal structures or continuing clinical suspicion. We have identified gene expression changes in stroma that can detect tumor nearby. We compared gene expression profiles of 13 biopsies containing stroma near tumor and 15 biopsies from volunteers without prostate cancer. About 3,800 significant expression changes were found and thereafter filtered using independent expression profiles to eliminate possible age-related genes and genes expressed at detectable levels in tumor cells. A stroma-specific classifier for nearby tumor was constructed on the basis of 114 candidate genes and tested on 364 independent samples including 243 tumor-bearing samples and 121 nontumor samples (normal biopsies, normal autopsies, remote stroma, as well as stroma within a few millimeters of tumor). The classifier predicted the tumor status of patients using tumor-free samples with an average accuracy of 97% (sensitivity = 98% and specificity = 88%) whereas classifiers trained with sets of 100 randomly generated genes had no diagnostic value. These results indicate that the prostate cancer microenvironment exhibits reproducible changes useful for categorizing the presence of tumor in patients when a prostate sample is derived from near the tumor but does not contain any recognizable tumor.
Figures
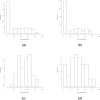
Figure 1
Histogram of tumor percentage for Datasets 1 – 4. The tumor percentage data of (a) and (b) were provided by SPECS pathologists, while the tumor percentage data of (c) and (d) were estimated by CellPred program (29). The stars in (a) mark the tumor percentages of the misclassified tumor-bearing cases in Dataset 1, which CellPred indicates may actually be non-tumor samples.
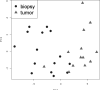
Figure 2
Plot of the Principal Component Analysis of training cases using the 131 probe-set Diagnostic Classifier.
Comment in
- Prostate cancer: biopsy samples from noncancerous stromal tissue can be used to diagnose nearby cancer.
Fenner A. Fenner A. Nat Rev Urol. 2011 Jun 10;8(6):294. doi: 10.1038/nrurol.2011.74. Nat Rev Urol. 2011. PMID: 21660074 No abstract available.
Similar articles
- Expression changes in the stroma of prostate cancer predict subsequent relapse.
Jia Z, Rahmatpanah FB, Chen X, Lernhardt W, Wang Y, Xia XQ, Sawyers A, Sutton M, McClelland M, Mercola D. Jia Z, et al. PLoS One. 2012;7(8):e41371. doi: 10.1371/journal.pone.0041371. Epub 2012 Aug 1. PLoS One. 2012. PMID: 22870216 Free PMC article. Clinical Trial. - Six stroma-based RNA markers diagnostic for prostate cancer in European-Americans validated at the RNA and protein levels in patients in China.
Zhu J, Pan C, Jiang J, Deng M, Gao H, Men B, McClelland M, Mercola D, Zhong WD, Jia Z. Zhu J, et al. Oncotarget. 2015 Jun 30;6(18):16757-65. doi: 10.18632/oncotarget.4430. Oncotarget. 2015. PMID: 26158290 Free PMC article. - Gene expressional changes in prostate fibroblasts from cancerous tissue.
Reinertsen T, Halgunset J, Viset T, Flatberg A, Haugsmoen LL, Skogseth H. Reinertsen T, et al. APMIS. 2012 Jul;120(7):558-71. doi: 10.1111/j.1600-0463.2011.02865.x. Epub 2012 Jan 25. APMIS. 2012. PMID: 22716211 - [Gene expression profiling in prostatic cancer].
Ernst T, Hergenhahn M, Kenzelmann M, Cohen CD, Ikinger U, Kretzler M, Hollstein M, Gröne HJ. Ernst T, et al. Verh Dtsch Ges Pathol. 2002;86:165-75. Verh Dtsch Ges Pathol. 2002. PMID: 12647366 Review. German. - Prostatic tumor stroma: a key player in cancer progression.
Taylor RA, Risbridger GP. Taylor RA, et al. Curr Cancer Drug Targets. 2008 Sep;8(6):490-7. doi: 10.2174/156800908785699351. Curr Cancer Drug Targets. 2008. PMID: 18781895 Review.
Cited by
- Alterations in the methylome of the stromal tumour microenvironment signal the presence and severity of prostate cancer.
Lawrence MG, Pidsley R, Niranjan B, Papargiris M, Pereira BA, Richards M, Teng L, Norden S, Ryan A, Frydenberg M, Stirzaker C, Taylor RA, Risbridger GP, Clark SJ. Lawrence MG, et al. Clin Epigenetics. 2020 Mar 18;12(1):48. doi: 10.1186/s13148-020-00836-2. Clin Epigenetics. 2020. PMID: 32188493 Free PMC article. - RNA expression differences in prostate tumors and tumor-adjacent stroma between Black and White Americans.
Rahmatpanah F, Robles G, Lilly M, Keane T, Kumar V, Mercola D, Randhawa P, McClelland M. Rahmatpanah F, et al. Oncotarget. 2021 Jul 20;12(15):1457-1469. doi: 10.18632/oncotarget.28024. eCollection 2021 Jul 20. Oncotarget. 2021. PMID: 34316327 Free PMC article. - Mast Cell-Derived SAMD14 Is a Novel Regulator of the Human Prostate Tumor Microenvironment.
Teng LKH, Pereira BA, Keerthikumar S, Huang C, Niranjan B, Lee SN, Richards M, Schittenhelm RB, Furic L, Goode DL, Lawrence MG, Taylor RA, Ellem SJ, Risbridger GP, Lister NL. Teng LKH, et al. Cancers (Basel). 2021 Mar 11;13(6):1237. doi: 10.3390/cancers13061237. Cancers (Basel). 2021. PMID: 33799802 Free PMC article. - Expression changes in the stroma of prostate cancer predict subsequent relapse.
Jia Z, Rahmatpanah FB, Chen X, Lernhardt W, Wang Y, Xia XQ, Sawyers A, Sutton M, McClelland M, Mercola D. Jia Z, et al. PLoS One. 2012;7(8):e41371. doi: 10.1371/journal.pone.0041371. Epub 2012 Aug 1. PLoS One. 2012. PMID: 22870216 Free PMC article. Clinical Trial. - DigSee: Disease gene search engine with evidence sentences (version cancer).
Kim J, So S, Lee HJ, Park JC, Kim JJ, Lee H. Kim J, et al. Nucleic Acids Res. 2013 Jul;41(Web Server issue):W510-7. doi: 10.1093/nar/gkt531. Epub 2013 Jun 12. Nucleic Acids Res. 2013. PMID: 23761452 Free PMC article.
References
- O'Dowd GJ, Miller MC, Orozco R, Veltri RW. Analysis of repeated biopsy results within 1 year after a noncancer diagnosis. Urology. 2000;55(4):553–9. - PubMed
- Che M, Sakr W, Grignon D. Pathologic features the urologist should expect on a prostate biopsy. Urol Oncol. 2003;21(2):153–61. - PubMed
- Pepe P, Aragona F. Saturation prostate needle biopsy and prostate cancer detection at initial and repeat evaluation. Urology. 2007;70(6):1131–5. - PubMed
- Andriole GL, Bullock TL, Belani JS, et al. Is there a better way to biopsy the prostate? Prospects for a novel transrectal systematic biopsy approach. Urology. 2007;70(6 Suppl):22–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 CA009054/CA/NCI NIH HHS/United States
- U01 CA114810-02/CA/NCI NIH HHS/United States
- P30 CA062203/CA/NCI NIH HHS/United States
- U01 CA1148102/CA/NCI NIH HHS/United States
- U01 CA152738-01/CA/NCI NIH HHS/United States
- R01 CA116161/CA/NCI NIH HHS/United States
- U01 CA114810/CA/NCI NIH HHS/United States
- U01 CA152738/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical