Targeting kidney mesangium by nanoparticles of defined size - PubMed (original) (raw)
Targeting kidney mesangium by nanoparticles of defined size
Chung Hang J Choi et al. Proc Natl Acad Sci U S A. 2011.
Abstract
Nanoparticles are being investigated for numerous medical applications and are showing potential as an emerging class of carriers for drug delivery. Investigations on how the physicochemical properties (e.g., size, surface charge, shape, and density of targeting ligands) of nanoparticles enable their ability to overcome biological barriers and reach designated cellular destinations in sufficient amounts to elicit biological efficacy are of interest. Despite proven success in nanoparticle accumulation at cellular locations and occurrence of downstream therapeutic effects (e.g., target gene inhibition) in a selected few organs such as tumor and liver, reports on effective delivery of engineered nanoparticles to other organs still remain scarce. Here, we show that nanoparticles of ~75 ± 25-nm diameters target the mesangium of the kidney. These data show the effects of particle diameter on targeting the mesangium of the kidney. Because many diseases originate from this area of the kidney, our findings establish design criteria for constructing nanoparticle-based therapeutics for targeting diseases that involve the mesangium of the kidney.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
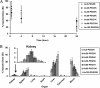
Fig. 1.
(A) Blood pharmacokinetics. All Au_x_-PEG_y_ NPs showed revealed extended circulation times in blood. (B) Organ-level biodistribution. Bulk particle localization in the liver, spleen, and kidney was size-dependent. Gold contents are normalized to percent injected dose (% ID). For all particle sizes, the five named organs plus the blood compartment accounted for at least 70% ID of the injected dose. Error bars indicate 1 SD from each Au_x_-PEG_y_ NP class (n = 3).
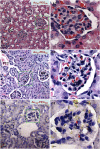
Fig. 2.
Tissue-level distribution in renal corpuscles within the cortex. Representative light micrographs of silver-enhanced kidney sections show the extent of glomerular targeting by particles. Au_x_-PEG_y_ NPs accumulated in a size-dependent manner. (A) Au20-PEG5,000 NPs were detectable in small quantities within renal corpuscles. (B) Au50-PEG5,000 NPs displayed the most intense staining in the largest area of renal corpuscles among all particle sizes. Silver staining (dark specks indicated by red arrows) was present in every single renal corpuscle observed under the light microscope, resulting in complete glomerular targeting efficiency (GTE). (C) Au100-PEG20,000 NPs only accumulated in the renal corpuscles in minute amounts, presumably because of their inability to penetrate through the fenestrated glomerular endothelium. Right illustrates the magnified renal corpuscle (green box) shown in Left. (Scale bar: Left, 10 μm; Right, 3 μm.) DC, distal convoluted tubule; PC, proximal convoluted tubule; PTC, peritubular capillaries; RC, renal corpuscle; U, urinary space.
Fig. 3.
Cellular-level distribution in renal corpuscles within the cortex. Representative transmission electron micrographs show particle accumulation in the mesangium (mesangial cells and extracellular matrix). Right illustrates the magnified portion (black box) shown in Left. (Scale bar: Left, 2 μm; Right, 500 nm.) Red arrows in Right indicate clusters of Au_x_-PEG_y_ NPs. (A) A small portion of Au20-PEG5,000 NPs localized in mesangial cells within the renal corpuscles. (B) Au50-PEG5,000 NPs experienced the most prominent uptake by mesangial cells among all particle sizes. (C) Au80-PEG10,000 NPs deposited in the mesangium in drastically reduced amounts. EC, endothelial cell; FP, foot processes of podocytes; GBM, glomerular basement membrane; MC, mesangial cell; PC, proximal convoluted tubule; Pe, parietal layer of Bowman's capsule; Po, podocyte; RBC, red blood cell; U, urinary space.
Similar articles
- Polymer decorated gold nanoparticles in nanomedicine conjugates.
Capek I. Capek I. Adv Colloid Interface Sci. 2017 Nov;249:386-399. doi: 10.1016/j.cis.2017.01.007. Epub 2017 Feb 15. Adv Colloid Interface Sci. 2017. PMID: 28259207 Review. - Gold nanoparticles as a versatile platform for optimizing physicochemical parameters for targeted drug delivery.
Bergen JM, von Recum HA, Goodman TT, Massey AP, Pun SH. Bergen JM, et al. Macromol Biosci. 2006 Jul 14;6(7):506-16. doi: 10.1002/mabi.200600075. Macromol Biosci. 2006. PMID: 16921538 - Functionalized gold nanoparticles manifested as potent carriers for nucleolar targeting.
Shahbazi R, Ozcicek I, Ozturk G, Ulubayram K. Shahbazi R, et al. Nanotechnology. 2017 Jan 13;28(2):025103. doi: 10.1088/1361-6528/28/2/025103. Epub 2016 Dec 7. Nanotechnology. 2017. PMID: 27924783 - siRNA delivery to the glomerular mesangium using polycationic cyclodextrin nanoparticles containing siRNA.
Zuckerman JE, Gale A, Wu P, Ma R, Davis ME. Zuckerman JE, et al. Nucleic Acid Ther. 2015 Apr;25(2):53-64. doi: 10.1089/nat.2014.0505. Epub 2015 Mar 3. Nucleic Acid Ther. 2015. PMID: 25734248 Free PMC article. - Multi-functional gold nanoparticles for drug delivery.
Han G, Ghosh P, Rotello VM. Han G, et al. Adv Exp Med Biol. 2007;620:48-56. doi: 10.1007/978-0-387-76713-0_4. Adv Exp Med Biol. 2007. PMID: 18217334 Review.
Cited by
- Role of Nanotechnology and Their Perspectives in the Treatment of Kidney Diseases.
Merlin JPJ, Li X. Merlin JPJ, et al. Front Genet. 2022 Jan 5;12:817974. doi: 10.3389/fgene.2021.817974. eCollection 2021. Front Genet. 2022. PMID: 35069707 Free PMC article. Review. - Thallium Labeled Citrate-Coated Prussian Blue Nanoparticles as Potential Imaging Agent.
Szigeti K, Hegedűs N, Rácz K, Horváth I, Veres DS, Szöllősi D, Futó I, Módos K, Bozó T, Karlinger K, Kovács N, Varga Z, Babos M, Budán F, Padmanabhan P, Gulyás B, Máthé D. Szigeti K, et al. Contrast Media Mol Imaging. 2018 Apr 26;2018:2023604. doi: 10.1155/2018/2023604. eCollection 2018. Contrast Media Mol Imaging. 2018. PMID: 29853803 Free PMC article. - Design and evaluation of glomerulus mesangium-targeted PEG-PLGA nanoparticles loaded with dexamethasone acetate.
Li S, Zeng YC, Peng K, Liu C, Zhang ZR, Zhang L. Li S, et al. Acta Pharmacol Sin. 2019 Jan;40(1):143-150. doi: 10.1038/s41401-018-0052-4. Epub 2018 Jun 27. Acta Pharmacol Sin. 2019. PMID: 29950614 Free PMC article. - Megalin-mediated specific uptake of chitosan/siRNA nanoparticles in mouse kidney proximal tubule epithelial cells enables AQP1 gene silencing.
Gao S, Hein S, Dagnæs-Hansen F, Weyer K, Yang C, Nielsen R, Christensen EI, Fenton RA, Kjems J. Gao S, et al. Theranostics. 2014 Aug 13;4(10):1039-51. doi: 10.7150/thno.7866. eCollection 2014. Theranostics. 2014. PMID: 25157280 Free PMC article. - Clinical translation of an ultrasmall inorganic optical-PET imaging nanoparticle probe.
Phillips E, Penate-Medina O, Zanzonico PB, Carvajal RD, Mohan P, Ye Y, Humm J, Gönen M, Kalaigian H, Schöder H, Strauss HW, Larson SM, Wiesner U, Bradbury MS. Phillips E, et al. Sci Transl Med. 2014 Oct 29;6(260):260ra149. doi: 10.1126/scitranslmed.3009524. Epub 2014 Oct 29. Sci Transl Med. 2014. PMID: 25355699 Free PMC article. Clinical Trial.
References
- Davis ME, Chen ZG, Shin DM. Nanoparticle therapeutics: An emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7:771–782. - PubMed
- Peer D, et al. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751–760. - PubMed
- Davis ME. The first targeted delivery of siRNA in humans via a self-assembling, cyclodextrin polymer-based nanoparticle: From concept to clinic. Mol Pharm. 2009;6:659–668. - PubMed
- Zimmermann TS, et al. RNAi-mediated gene silencing in non-human primates. Nature. 2006;441:111–114. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources