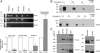Expression and roles of pannexins in ATP release in the pituitary gland - PubMed (original) (raw)
Expression and roles of pannexins in ATP release in the pituitary gland
Shuo Li et al. Endocrinology. 2011 Jun.
Abstract
Pannexins are a newly discovered three-member family of proteins expressed in the brain and peripheral tissues that belong to the superfamily of gap junction proteins. However, in mammals pannexins do not form gap junctions, and their expression and function in the pituitary gland have not been studied. Here we show that the rat pituitary gland expresses mRNA and protein transcripts of pannexins 1 and 2 but not pannexin 3. Pannexin 1 was more abundantly expressed in the anterior lobe, whereas pannexin 2 was more abundantly expressed in the intermediate and posterior pituitary. Pannexin 1 was identified in corticotrophs and a fraction of somatotrophs, the S100-positive pituicytes of the posterior pituitary and AtT-20 (mouse pituitary adrenocorticotropin-secreting cells) and rat immortalized pituitary cells secreting prolactin, whereas pannexin 2 was detected in the S100-positive folliculostellate cells of the anterior pituitary, melanotrophs of the intermediate lobe, and vasopressin-containing axons and nerve endings in the posterior lobe. Overexpression of pannexins 1 and 2 in AtT-20 pituitary cells enhanced the release of ATP in the extracellular medium, which was blocked by the gap junction inhibitor carbenoxolone. Basal ATP release in At-T20 cells was also suppressed by down-regulating the expression of endogenous pannexin 1 but not pannexin 2 with their short interfering RNAs. These results indicate that pannexins may provide a pathway for delivery of ATP, which is a native agonist for numerous P2X cationic channels and G protein-coupled P2Y receptors endogenously expressed in the pituitary gland.
Figures
Fig. 1.
Identification of Panx transcripts in rat pituitary cells and cell lines. The primer pairs Panx1F2/Panx1R2, Panx2F1/Panx2R1, and Panx3F/Panx3R were used for detection of Panx1, Panx2, and Panx3 mRNAs, respectively (Table 1). A, inset, The examination of Panx1, Panx2, and Panx3 mRNA transcripts in pituitary tissue and various cell lines by RT-PCR. The sizes of PCR products were as follows: 548 bp for rat Panx1, 786 bp for rat Panx2, and 372 bp for rat Panx3. PCR was performed using first-strand cDNA samples with (+) and without (−) reverse transcriptase, and agarose gel was performed to analyze the PCR products. The identity of all PCR products was confirmed by DNA sequencing. No PCR products were detected in controls containing all components except the reverse transcriptase. A, main panel, Real-time RT-PCR analysis of Panx gene expression in rat anterior and intermediate-posterior pituitary tissues, GH3, and AtT-20 pituitary cell lines. Panx1 gene expression was set as 100% and Panx2 gene expression was therefore presented as a normalized value. Panx1 and Panx2 genes show significantly different levels of expression in different tissues and cells. *, P < 0.01, estimated by Student's t test. B, Characterization of Panx antibodies by Western blot analysis. HEK293 cells were transfected (+) or untransfected (−) with Panx1 or Panx2 plasmid, and 5 μg (transfected) or 50 μg (untransfected) cell lysates were applied onto each well of gels. The blot membranes were incubated with different dilutions of anti-Panx antibodies, developed, and exposed under the same conditions. The specific bands for both Panx1 and Panx2 were completely eliminated when the anti-Panx antibodies were preadsorbed with their respectively cognate peptides by 1:1 weight ratio. C, Expression of Panx1 and Panx2 proteins in rat pituitary cells. Approximately 50 μg total proteins from each cell type was loaded onto each well of gels and Western blots were performed using rabbit anti-Panx1 and -Panx2 antibodies (0.5 μg/ml). Panx1 and Panx2 proteins were expressed in cultured pituitary cells, AtT-20, and GH3 immortalized AP cells but not in HEK293 cells (left panel). The upper band for Panx1 (∼100 kDa) in GH3 cells represents a dimer. Panx2 shows a single band in AtT-20 pituitary cells but displays two bands in pituitary cells as well as in GH3 pituitary cells. Recombinant V5-tagged Panx1 or Panx2 protein serves as a control to confirm the identities of endogenous expressed Panx (right panels). Note that the ectopically expressed Panx1 protein shows three bands that represent different glycosylated forms, whereas Panx1 protein from rat pituitary cells presents only one glycosylated form. In this and the following figures, triplicate experiments were performed and similar results were obtained.
Fig. 2.
Oligomeric organization of Panx channels. HEK293 cells were transfected with FLAG-tagged Panx1 (A and C) or V5-tagged Panx1 and Panx2 constructs (B). Proteins from the pituitary tissue and transfected HEK293 cells were extracted with PBS containing 1% digitonin, and Panx1 and Panx2 oligomers were evaluated under native conditions (for details see Materials and Methods). A, Assembly of Panx1 homomeric oligomers in HEK293 cells and pituitary tissue. B, Assembly of Panx2 homomeric oligomers in HEK293 cells. C and D, Characterization of Panx1 protein oligomeric complexes in HEK293 cells (C) and pituitary tissue (D) by SDS treatment. Proteins were immunoblotted with anti-Panx1 (A and D), anti-V5 antibody (B), or anti-FLAG horseradish peroxidase-labeled antibody (C). Note that SDS-treated Panx1 oligomeric complexes from HEK293 cells can partially dissociate into three additional bands, probably representing trimeric (three times), dimeric (two times), and monomeric (one time) forms, whereas Panx1 oligomeric complexes from pituitary tissue can only partially dissociate into dimeric and monomeric forms through SDS treatment. The molecular-weight protein markers are shown on the left sides of the panels, which may not represent the actual size of the proteins (52).
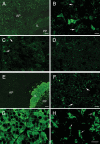
Fig. 3.
Localization of Panx1 and Panx2 proteins in the rat pituitary gland. Immunofluorescence labeling for Panx1 (A–D) and Panx2 (E–H) in different lobes of the pituitary gland. A and E, Low-magnification immunofluorescence images showing the overall distribution of Panx1 (A) and Panx2 (E) in the AP, IL, and PP, respectively. B, In the AP, anti-Panx1 strongly labeled cells with irregular shapes that morphologically resembled corticotrophs. Panx1 also displayed a dot-like cytoplasmic and membranous distribution in other cells of the AP (arrows). C, In the IL, the expression of Panx1 was low, with the exception of the epithelial cells bordering the pituitary cleft, which were moderately immunolabeled (arrows). D, In the PP, Panx1 displayed punctate and diffuse distribution, without distinguishable cellular structures. F, In the AP, Panx2 showed punctate, cytoplasmatic, and membranous distribution pattern (arrows). G, Panx2 was strongly and intensely expressed in endocrine cells throughout the IL (melanotrophs). H, In the PP, Panx2 showed intensive immunostaining in round or ovoid-shaped presumably neuronal structures with variable dimensions (arrows). Scale bars (A and E), 100 μm; (B–D, F, and G), 10 μm; (H), 50 μm.
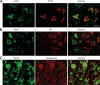
Fig. 4.
Double-immunofluorescence labeling of Panx in AP and PP. A, Panx1 (green fluorescence) was highly expressed by ACTH-containing cells (red fluorescence). B, Panx1 (green fluorescence) showed punctate staining in GH-positive cells (red fluorescence). C, Panx2 (green fluorescence) is expressed by most of the vasopressin-containing axonal elements (red fluorescence). Scale bar (applies to all images), 10 μm.
Fig. 5.
Double-immunofluorescence labeling of Panx and S100 protein in the AP and PP. A, Panx1 (green fluorescence) was associated with S100-positive pituicytes (red florescence) in the PP (arrows). B, Panx2 (green fluorescence) was observed in S100-positive (red florescence) foliculostellate cells in the AP (arrows). C, Negative controls of immunolabeling did not result in specific labeling. Scale bar (applies to all images), 5 μm.
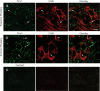
Fig. 6.
Immunofluorescence labeling of Panx in cultured AP cells. The presence of Panx1 (A) and Panx2 (C) in a fraction of cells is shown. The immunolableing for both Panx1 (B) and Panx2 (D) was completely eliminated when the anti-Panx antibodies were preadsorbed with their respectively cognate peptides by 1:1 weight ratio. E–G, Double immunocytochemistry of ACTH and Panx1 in primary AP cells. Representative images show ACTH-labeled cells (E, red fluorescence, arrows), Panx1 immunopositive cells (F, green fluorescence), and merged image (G). 4′,6′-Diamino-2-phenylindole (blue fluorescence) denotes the nuclei of cells in all panels.
Fig. 7.
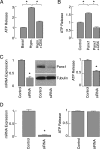
Panx1-mediated extracellular ATP release in AtT-20 pituitary cells. A, Basal and hypotonic (hypo)-induced ATP release in the presence and absence of 100 μ
m
CBX. Results were normalized with respect to basal ATP release. B, Effects of overexpression of Panx1 on hypotonic-induced ATP release in the presence and absence of 100 μ
m
CBX. Results were normalized with respect to hypotonically induced ATP release by cells transfected with control plasmid. C, left panels, Down-regulation of endogenous Panx1 expression by Panx1 siRNA in AtT-20 cells. These cells were transfected with control siRNA or Panx1 siRNA and the knockdown of endogenous Panx1 expression was examined by real-time PCR (left panels, 48 h after transfection) and Western blot (right panels, 72 h after transfection). C, right panels, Inhibition of ATP release by silencing the endogenously expressed Panx1 with Panx1 siRNA (after 72 h incubation). D, The lack of effect of silencing Panx2 expression on ATP release. Data shown are normalized with respect to controls and are shown as mean ±
sem
values. *, P < 0.001, estimated by Student's t test.
Similar articles
- Mechanosensitive release of adenosine 5'-triphosphate through pannexin channels and mechanosensitive upregulation of pannexin channels in optic nerve head astrocytes: a mechanism for purinergic involvement in chronic strain.
Beckel JM, Argall AJ, Lim JC, Xia J, Lu W, Coffey EE, Macarak EJ, Shahidullah M, Delamere NA, Zode GS, Sheffield VC, Shestopalov VI, Laties AM, Mitchell CH. Beckel JM, et al. Glia. 2014 Sep;62(9):1486-501. doi: 10.1002/glia.22695. Epub 2014 May 19. Glia. 2014. PMID: 24839011 Free PMC article. - Characterization of novel Pannexin 1 isoforms from rat pituitary cells and their association with ATP-gated P2X channels.
Li S, Tomić M, Stojilkovic SS. Li S, et al. Gen Comp Endocrinol. 2011 Nov 1;174(2):202-10. doi: 10.1016/j.ygcen.2011.08.019. Epub 2011 Sep 1. Gen Comp Endocrinol. 2011. PMID: 21907716 Free PMC article. - Pannexin 1 channels mediate the release of ATP into the lumen of the rat urinary bladder.
Beckel JM, Daugherty SL, Tyagi P, Wolf-Johnston AS, Birder LA, Mitchell CH, de Groat WC. Beckel JM, et al. J Physiol. 2015 Apr 15;593(8):1857-71. doi: 10.1113/jphysiol.2014.283119. Epub 2015 Feb 11. J Physiol. 2015. PMID: 25630792 Free PMC article. - Signaling by purinergic receptors and channels in the pituitary gland.
Stojilkovic SS, He ML, Koshimizu TA, Balik A, Zemkova H. Stojilkovic SS, et al. Mol Cell Endocrinol. 2010 Jan 27;314(2):184-91. doi: 10.1016/j.mce.2009.05.008. Epub 2009 May 23. Mol Cell Endocrinol. 2010. PMID: 19467293 Free PMC article. Review. - Pannexin-1 as a potentiator of ligand-gated receptor signaling.
Isakson BE, Thompson RJ. Isakson BE, et al. Channels (Austin). 2014;8(2):118-23. doi: 10.4161/chan.27978. Epub 2014 Feb 27. Channels (Austin). 2014. PMID: 24576994 Free PMC article. Review.
Cited by
- Vitamin D3 Treatment Alters Thyroid Functional Morphology in Orchidectomized Rat Model of Osteoporosis.
Šošić-Jurjević B, Trifunović S, Živanović J, Ajdžanović V, Miler M, Ristić N, Filipović B. Šošić-Jurjević B, et al. Int J Mol Sci. 2022 Jan 12;23(2):791. doi: 10.3390/ijms23020791. Int J Mol Sci. 2022. PMID: 35054977 Free PMC article. - Pannexin 1 channels and ATP release in epilepsy: two sides of the same coin : The contribution of pannexin-1, connexins, and CALHM ATP-release channels to purinergic signaling.
Dossi E, Rouach N. Dossi E, et al. Purinergic Signal. 2021 Dec;17(4):533-548. doi: 10.1007/s11302-021-09818-2. Epub 2021 Sep 8. Purinergic Signal. 2021. PMID: 34495463 Free PMC article. Review. - Structure of the full-length human Pannexin1 channel and insights into its role in pyroptosis.
Zhang S, Yuan B, Lam JH, Zhou J, Zhou X, Ramos-Mandujano G, Tian X, Liu Y, Han R, Li Y, Gao X, Li M, Yang M. Zhang S, et al. Cell Discov. 2021 May 4;7(1):30. doi: 10.1038/s41421-021-00259-0. Cell Discov. 2021. PMID: 33947837 Free PMC article. - Cell Type- and Sex-Dependent Transcriptome Profiles of Rat Anterior Pituitary Cells.
Fletcher PA, Smiljanic K, Maso Prévide R, Iben JR, Li T, Rokic MB, Sherman A, Coon SL, Stojilkovic SS. Fletcher PA, et al. Front Endocrinol (Lausanne). 2019 Sep 18;10:623. doi: 10.3389/fendo.2019.00623. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 31620083 Free PMC article. - Distinct Expression Patterns of Osteopontin and Dentin Matrix Protein 1 Genes in Pituitary Gonadotrophs.
Bjelobaba I, Janjic MM, Prévide RM, Abebe D, Kucka M, Stojilkovic SS. Bjelobaba I, et al. Front Endocrinol (Lausanne). 2019 Apr 17;10:248. doi: 10.3389/fendo.2019.00248. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 31057484 Free PMC article.
References
- Ralevic V, Burnstock G. 1998. Receptors for purines and pyrimidines. Pharmacol Rev 50:413–492 - PubMed
- Knott TK, Marrero HG, Custer EE, Lemos JR. 2008. Endogenous ATP potentiates only vasopressin secretion from neurohypophysial terminals. J Cell Physiol 217:155–161 - PubMed
- Burnstock G, Knight GE. 2004. Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol 240:31–304 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous