Growth hormone and adipose tissue: beyond the adipocyte - PubMed (original) (raw)
Review
Growth hormone and adipose tissue: beyond the adipocyte
Darlene E Berryman et al. Growth Horm IGF Res. 2011 Jun.
Abstract
The last two decades have seen resurgence in research focused on adipose tissue. In part, the enhanced interest stems from an alarming increase in obesity rates worldwide. However, an understanding that this once simple tissue is significantly more intricate and interactive than previously realized has fostered additional attention. While few would argue that growth hormone (GH) radically alters fat mass, newer findings revealing the complexity of adipose tissue requires that GH's influence on this tissue be reexamined. Therefore, the objective of this review is to describe the more recent understanding of adipose tissue and to summarize our current knowledge of how GH may influence and contribute to these newer complexities of this tissue with special focus on the available data from mice with altered GH action.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures
Fig. 1
Commonly studied mouse adipose depots. Shown are the location and main characteristics of several WAT depots studied in male mice. The mesenteric fat pad (top left), which is intertwined along the intestines, is a true visceral fat pad. The inguinal fat pad (bottom left), which is characterized by the inguinal lymph node, routinely is used to represent SubQ fat although other SubQ fat pads can be isolated (i.e. subscapular, not shown). Inset image shows the abdomen after dissection of the intestines, to view the kidney (K) and the retroperitoneal depot (top right). There are two fat pads surrounding the kidney (retroperitoneal and perirenal). In some cases, the perirenal and retroperitoneal fat pads can be separated but often are not easily distinguishable at least in younger mice; therefore, many researchers isolate both collectively and use the names for these distinct fat pads interchangeably. Shown is the retroperitoneal fat pad, which we routinely isolate and is behind the kidney. The perirenal fat pad is below and around the kidney (not shown). Epididymal depots (bottom right) have been moved outside of the abdomen while conserving their attachment point. Epididymal fat pads are the most often studied depot because they are easy to isolate and typically the largest in mass. Their classification as visceral depends on the definition used (i.e. these pads do not drain into the portal vein), as discussed in the text. The comparable fat pad in female mice surrounds the ovaries and is called the periovarian depot. Because of their close proximity to gonadal tissue, the epididymal and periovarian fat pads can also be called perigonadal fat, a gender neutral term. (Adapted from Sackmann-Sala et al., submitted).
Fig. 2
Schematic representation of depot-specific differences between SubQ and intra-abdominal adipose tissue. SubQ adipose tissue has adipocytes with more extremes in adipocyte size (shown in this figure as smaller, as has been reported for lean, younger rodents; however, these adipocytes have a greater potential to expand, resulting in larger adipocytes in obese states), has a greater potential for preadipocyte differentiation and senescence (as noted by blue shading within the preadipocyte) [35, 36], has greater potential to convert progenitor cells into brown adipocytes [12] as well as more abundant and organized collagen fibers [125]. In contrast, several intra-abdominal depots are considered to be more vascularized and have greater immune cell content. These differences are often coupled with or potentially caused by differences in hormone receptor numbers.
Fig. 3
Image of wild-type mice, giant bGH transgenic, dwarf GHA transgenic and dwarf GHR-/- gene disrupted mice in the same genetic background (C57BL/6J). These mice represent normal, elevated, decreased and absent levels of GH action, respectively.
Fig. 4
Percent body fat for male and female bGH and WT mice. Data are expressed as mean ± SEM, n = 7 (male bGH), n = 8 (male WT), and n = 10 (female bGH and WT). Repeated-measures ANOVA test reveals a significant effect of gender [F(1,31) = 19.1, P < 0.001] and genotype [F(1,31) = 6.2, P = 0.02] as well as a significant interaction between genotype and gender [F(1,31) = 5.8, P = 0.02]. Adapted from Palmer et al. [77]
Fig. 5
Hematoxylin and eosin staining of inguinal SubQ and epididymal adipose tissue. Adipose tissue samples were obtained from 6 month old GHR-/-, GHA, bGH and control mice. Adipose tissue was fixed in 10% buffered formalin, paraffin-embedded and then sections of 5 μm were stained.
Fig. 6
Longitudinal comparison of epididymal, retroperitoneal and inguinal SubQ adipose depots in giant bGH mice (top) and dwarf GHR-/- mice (bottom). Due to a significantly shorter lifespan, depot weights were not collected at later time points for the bGH mice. All depots are similarly impacted in bGH mice although an age-dependent difference is apparent. Despite the extreme dwarf size of GHR-/- mice, the absolute mass of the SubQ depot (bottom right) is larger than that of WT mice at all ages. In contrast, the epididymal fat pad is proportional to the dwarf size of the animal at all ages (bottom left). For the retroperitoneal fat pad, there is no significant difference at any age (bottom center). Thus, there is a significant depot-specific difference in depot size in GHR-/- mice.
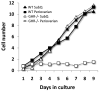
Fig 7
Proliferation of stromal vascular cells derived from WT and GHR-/- mice. Stromal vascular cells from five month old female mice were used. Preadipocytes isolated from SubQ and periovarian adipose tissue derived from WT mice or from SubQ adipose tissue from GHR-/- mice proliferate normally; however, preadipocytes derived from periovarian adipose tissue from GHR-/- mice fail to proliferate in culture. Figure adapted from Flint et al. [100]
Similar articles
- Growth Hormone's Effect on Adipose Tissue: Quality versus Quantity.
Berryman DE, List EO. Berryman DE, et al. Int J Mol Sci. 2017 Jul 26;18(8):1621. doi: 10.3390/ijms18081621. Int J Mol Sci. 2017. PMID: 28933734 Free PMC article. Review. - Growth hormone (GH) differentially regulates NF-kB activity in preadipocytes and macrophages: implications for GH's role in adipose tissue homeostasis in obesity.
Kumar PA, Chitra PS, Lu C, Sobhanaditya J, Menon R. Kumar PA, et al. J Physiol Biochem. 2014 Jun;70(2):433-40. doi: 10.1007/s13105-014-0321-8. Epub 2014 Feb 15. J Physiol Biochem. 2014. PMID: 24532264 - The role of GH in adipose tissue: lessons from adipose-specific GH receptor gene-disrupted mice.
List EO, Berryman DE, Funk K, Gosney ES, Jara A, Kelder B, Wang X, Kutz L, Troike K, Lozier N, Mikula V, Lubbers ER, Zhang H, Vesel C, Junnila RK, Frank SJ, Masternak MM, Bartke A, Kopchick JJ. List EO, et al. Mol Endocrinol. 2013 Mar;27(3):524-35. doi: 10.1210/me.2012-1330. Epub 2013 Jan 24. Mol Endocrinol. 2013. PMID: 23349524 Free PMC article. - The effects of growth hormone on adipose tissue: old observations, new mechanisms.
Kopchick JJ, Berryman DE, Puri V, Lee KY, Jorgensen JOL. Kopchick JJ, et al. Nat Rev Endocrinol. 2020 Mar;16(3):135-146. doi: 10.1038/s41574-019-0280-9. Epub 2019 Nov 28. Nat Rev Endocrinol. 2020. PMID: 31780780 Free PMC article. Review. - GH Knockout Mice Have Increased Subcutaneous Adipose Tissue With Decreased Fibrosis and Enhanced Insulin Sensitivity.
List EO, Berryman DE, Buchman M, Jensen EA, Funk K, Duran-Ortiz S, Qian Y, Young JA, Slyby J, McKenna S, Kopchick JJ. List EO, et al. Endocrinology. 2019 Jul 1;160(7):1743-1756. doi: 10.1210/en.2019-00167. Endocrinology. 2019. PMID: 31099824 Free PMC article.
Cited by
- Growth hormone and IGF-1 deficiency exacerbate high-fat diet-induced endothelial impairment in obese Lewis dwarf rats: implications for vascular aging.
Bailey-Downs LC, Sosnowska D, Toth P, Mitschelen M, Gautam T, Henthorn JC, Ballabh P, Koller A, Farley JA, Sonntag WE, Csiszar A, Ungvari Z. Bailey-Downs LC, et al. J Gerontol A Biol Sci Med Sci. 2012 Jun;67(6):553-64. doi: 10.1093/gerona/glr197. Epub 2011 Nov 10. J Gerontol A Biol Sci Med Sci. 2012. PMID: 22080499 Free PMC article. - Liver-specific GH receptor gene-disrupted (LiGHRKO) mice have decreased endocrine IGF-I, increased local IGF-I, and altered body size, body composition, and adipokine profiles.
List EO, Berryman DE, Funk K, Jara A, Kelder B, Wang F, Stout MB, Zhi X, Sun L, White TA, LeBrasseur NK, Pirtskhalava T, Tchkonia T, Jensen EA, Zhang W, Masternak MM, Kirkland JL, Miller RA, Bartke A, Kopchick JJ. List EO, et al. Endocrinology. 2014 May;155(5):1793-805. doi: 10.1210/en.2013-2086. Epub 2014 Feb 11. Endocrinology. 2014. PMID: 24517230 Free PMC article. - Altered structure and function of adipose tissue in long-lived mice with growth hormone-related mutations.
Darcy J, McFadden S, Bartke A. Darcy J, et al. Adipocyte. 2017 Apr 3;6(2):69-75. doi: 10.1080/21623945.2017.1308990. Epub 2017 Mar 21. Adipocyte. 2017. PMID: 28425851 Free PMC article. Review. - The impact of growth hormone on proteomic profiles: a review of mouse and adult human studies.
Duran-Ortiz S, Brittain AL, Kopchick JJ. Duran-Ortiz S, et al. Clin Proteomics. 2017 Jun 29;14:24. doi: 10.1186/s12014-017-9160-2. eCollection 2017. Clin Proteomics. 2017. PMID: 28670222 Free PMC article. Review. - GH action influences adipogenesis of mouse adipose tissue-derived mesenchymal stem cells.
Olarescu NC, Berryman DE, Householder LA, Lubbers ER, List EO, Benencia F, Kopchick JJ, Bollerslev J. Olarescu NC, et al. J Endocrinol. 2015 Jul;226(1):13-23. doi: 10.1530/JOE-15-0012. Epub 2015 May 5. J Endocrinol. 2015. PMID: 25943560 Free PMC article.
References
- Cinti S. In: Morphology of the Adipose Organ, in Adipose tissue. Klaus S, editor. Landes Bioscience; Austin, Texas: 2001. pp. 11–26.
- Klaus S. Adipose tissue as a regulator of energy balance. Curr Drug Targets. 2004;5:241–250. - PubMed
- Ailhaud G, Grimaldi P, Negrel R. Cellular and molecular aspects of adipose tissue development. Annu Rev Nutr. 1992;12:207–233. - PubMed
- Ailhaud G. In: Development of White Adipose Tissue and Adipocyte Differentiation, in Adipose tissue. Klaus S, editor. Austin, Texas: Eurekah.com, Georgetown; Texas: Landes Bioscience; 2001. p. 27.
- Ravussin E. The presence and role of brown fat in adult humans. Curr Diab Rep. 2010;10:90–92. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources