Interpreting patient-reported outcome results: US FDA guidance and emerging methods - PubMed (original) (raw)
Review
Interpreting patient-reported outcome results: US FDA guidance and emerging methods
Lori D McLeod et al. Expert Rev Pharmacoecon Outcomes Res. 2011 Apr.
Abstract
In recent years, the US FDA has become more critical of instruments used to measure patient-reported outcomes (PROs) in clinical trials. To facilitate decisions related to the approval of drugs, labels and promotional claims based on PROs, the FDA created the Study Endpoints and Label Development (SEALD) group. SEALD has developed a PRO guidance related to the use of PRO measures used to support drug approvals and label claims, including recommendations for establishing thresholds for meaningful change at the individual level (i.e., defining a responder). This article examines in detail the FDA-recommended methodology for defining a responder and analyzing responder-based PRO measure results. We also present other responder analysis approaches for consideration in furthering the precision and interpretation of this methodology.
Figures
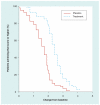
Figure 1. Sample cumulative distribution function
The data used to draw this figure were generated for illustration purposes.
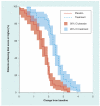
Figure 2. Sample cumulative distribution function with confidence bands
The data used to draw this figure were generated for illustration purposes. CI: Confidence interval.
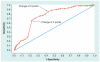
Figure 3. Sample receiver operating characteristic curve
Data from [14].
Similar articles
- Methods for interpreting change over time in patient-reported outcome measures.
Wyrwich KW, Norquist JM, Lenderking WR, Acaster S; Industry Advisory Committee of International Society for Quality of Life Research (ISOQOL). Wyrwich KW, et al. Qual Life Res. 2013 Apr;22(3):475-83. doi: 10.1007/s11136-012-0175-x. Epub 2012 Apr 17. Qual Life Res. 2013. PMID: 22528240 Review. - Potential of patient-reported outcomes as nonprimary endpoints in clinical trials.
Gnanasakthy A, Lewis S, Clark M, Mordin M, DeMuro C. Gnanasakthy A, et al. Health Qual Life Outcomes. 2013 May 15;11:83. doi: 10.1186/1477-7525-11-83. Health Qual Life Outcomes. 2013. PMID: 23675876 Free PMC article. - US FDA patient-reported outcome guidance: great expectations and unintended consequences.
Fehnel S, DeMuro C, McLeod L, Coon C, Gnanasakthy A. Fehnel S, et al. Expert Rev Pharmacoecon Outcomes Res. 2013 Aug;13(4):441-6. doi: 10.1586/14737167.2013.814957. Expert Rev Pharmacoecon Outcomes Res. 2013. PMID: 23977972 Review. - Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance.
U.S. Department of Health and Human Services FDA Center for Drug Evaluation and Research; U.S. Department of Health and Human Services FDA Center for Biologics Evaluation and Research; U.S. Department of Health and Human Services FDA Center for Devices and Radiological Health. U.S. Department of Health and Human Services FDA Center for Drug Evaluation and Research, et al. Health Qual Life Outcomes. 2006 Oct 11;4:79. doi: 10.1186/1477-7525-4-79. Health Qual Life Outcomes. 2006. PMID: 17034633 Free PMC article. - Patient Reported Outcome (PRO) assessment in epilepsy: a review of epilepsy-specific PROs according to the Food and Drug Administration (FDA) regulatory requirements.
Nixon A, Kerr C, Breheny K, Wild D. Nixon A, et al. Health Qual Life Outcomes. 2013 Mar 11;11:38. doi: 10.1186/1477-7525-11-38. Health Qual Life Outcomes. 2013. PMID: 23497117 Free PMC article. Review.
Cited by
- Pragmatic Implementation of a Stratified Primary Care Model for Low Back Pain Management in Outpatient Physical Therapy Settings: Two-Phase, Sequential Preliminary Study.
Beneciuk JM, George SZ. Beneciuk JM, et al. Phys Ther. 2015 Aug;95(8):1120-34. doi: 10.2522/ptj.20140418. Epub 2015 Apr 9. Phys Ther. 2015. PMID: 25858972 Free PMC article. Clinical Trial. - Biologic Abatement and Capturing Kids' Outcomes and Flare Frequency in Juvenile Spondyloarthritis (BACK-OFF JSpA): study protocol for a randomized pragmatic trial.
Weiss PF, Sears CE, Brandon TG, Forrest CB, Neu E, Kohlheim M, Leal J, Xiao R, Lovell D. Weiss PF, et al. Trials. 2023 Feb 8;24(1):100. doi: 10.1186/s13063-022-07038-6. Trials. 2023. PMID: 36755328 Free PMC article. - The Need for a Developmentally Based Measure of Social Communication Skills.
Bishop S, Farmer C, Kaat A, Georgiades S, Kanne S, Thurm A. Bishop S, et al. J Am Acad Child Adolesc Psychiatry. 2019 Jun;58(6):555-560. doi: 10.1016/j.jaac.2018.12.010. J Am Acad Child Adolesc Psychiatry. 2019. PMID: 31130206 Free PMC article. - Development and Validation of a Juvenile Spondyloarthritis Disease Flare Measure: Ascertaining Flare in Patients With Inactive Disease.
Weiss PF, Brandon TG, Ryan ME, Treemarcki EB, Armendariz S, Wright TB, Godiwala C, Stoll ML, Xiao R, Lovell D. Weiss PF, et al. Arthritis Care Res (Hoboken). 2023 Feb;75(2):373-380. doi: 10.1002/acr.24763. Epub 2022 Sep 13. Arthritis Care Res (Hoboken). 2023. PMID: 34363343 Free PMC article. - The Definition of Failure in Hip Arthroscopy May Include Factors Outside of Reoperation: A Systematic Review.
Bernard CD, Bowles E, Trotter M, Aldag L, Henkelman E, Long R, Schroeppel P, Mullen S, White J, Tarakemeh A, Vopat B. Bernard CD, et al. Arthrosc Sports Med Rehabil. 2024 Jun 26;6(5):100962. doi: 10.1016/j.asmr.2024.100962. eCollection 2024 Oct. Arthrosc Sports Med Rehabil. 2024. PMID: 39534025 Free PMC article. Review.
References
- Juniper ER, Guyatt GH, Willan A, Griffith LE. Determining a minimal important change in a disease-specific quality of life questionnaire. J. Clin. Epidemiol. 1994;47(1):81–87. •• Provides a description of the anchor-based approach to identifying a minimal important change for a patient-reported outcome (PRO) measure.
- Hays RD, Farivar SS, Liu H. Approaches and recommendations for estimating minimally important differences for health-related quality of life measures. COPD: J. Chron. Obstruct. Pulmon. Dis. 2005;2:63–67. - PubMed
- Wyrwich KW, Tierney WM, Wolinsky FD. Further evidence supporting an SEM-based criterion for identifying meaningful intra-individual changes in health-related quality of life. J. Clin. Epidemiol. 1999;52(9):861–873. •• Provides a description of the the standard error of measurement-based approach to identifying a minimal important change for a PRO measure.
- Farrar JT, Dworkin RH, Max MB. Use of the cumulative proportion of responders analysis graph to present pain data over a range of cutoff points: making clinical trial data more understandable. J. Pain Symptom Manage. 2006;31(4):369–377. •• Provides a working example of how a cumulative distribution function can be used to evaluate a range of responder cutoff points for an 11-point numerical pain rating scale.
Websites
- US Department of Health and Human Services (USDHHS) [Accessed 1 December 2010];Draft guidance for industry. Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. 2006 February; www.ispor.org/workpaper/FDAPROGuidance2006.pdf. - PMC - PubMed
- US Department of Health and Human Services (USDHHS) [Accessed 1 December 2010];Guidance for industry. Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. 2009 December; www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatory Information/Guidances/UCM193282.pdf. •• US FDA guidance for industry related to the development and review of PRO measures.
Publication types
MeSH terms
Grants and funding
- P30-AG028748/AG/NIA NIH HHS/United States
- P30 AG021684-04/AG/NIA NIH HHS/United States
- 2P20MD000182/MD/NIMHD NIH HHS/United States
- P20 MD000182-10/MD/NIMHD NIH HHS/United States
- P20 MD000182/MD/NIMHD NIH HHS/United States
- P30 AG028748-05/AG/NIA NIH HHS/United States
- P30 AG021684/AG/NIA NIH HHS/United States
- P30-AG021684/AG/NIA NIH HHS/United States
- P30 AG028748/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous