Identification of a novel Bcl-2-interacting mediator of cell death (Bim) E3 ligase, tripartite motif-containing protein 2 (TRIM2), and its role in rapid ischemic tolerance-induced neuroprotection - PubMed (original) (raw)
Identification of a novel Bcl-2-interacting mediator of cell death (Bim) E3 ligase, tripartite motif-containing protein 2 (TRIM2), and its role in rapid ischemic tolerance-induced neuroprotection
Simon Thompson et al. J Biol Chem. 2011.
Abstract
We have previously shown that the cell death-promoting protein Bcl-2-interacting mediator of cell death (Bim) is ubiquitinated and degraded following a neuroprotection-conferring episode of brief ischemia (preconditioning). Here, we identify the E3 ligase that ubiquitinates Bim in this model, using a proteomics approach. Using phosphorylated GST-Bim as bait, we precipitated and identified by mass spectrometry tripartite motif protein 2 (TRIM2), a RING (really interesting new gene) domain-containing protein. The reaction between TRIM2 and Bim was confirmed using co-immunoprecipitation followed by immunoblotting. We show that TRIM2 binds to Bim when it is phosphorylated by p42/p44 MAPK but does not interact with a nonphosphorylatable Bim mutant (3ABim). 12-O-tetradecanoylphorbol-13-acetate activation of p42/p44 MAPK drives Bim ubiquitination in mouse embryonic fibroblast cells and is associated with an increased interaction between TRIM2 and Bim. One hour following preconditioning ischemia, the binding of Bim to TRIM2 increased, consistent with the time window of enhanced Bim degradation. Blocking p42/p44 MAPK activation following preconditioning ischemia with U0126 or using the nonphosphorylatable 3ABim reduced the binding between Bim and TRIM2. Immunodepletion of TRIM2 from cell lysates prepared from preconditioned cells reduced Bim ubiquitination. Finally, suppression of TRIM2 expression, using lentivirus transduction of shRNAmir, stabilized Bim protein levels and blocked neuroprotection observed in rapid ischemic tolerance. Taken together, these data support a role for TRIM2 in mediating the p42/p44 MAPK-dependent ubiquitination of Bim in rapid ischemic tolerance.
Figures
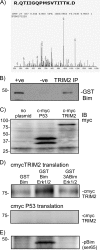
FIGURE 1.
Proteomic identification of TRIM2 as a Bim-binding protein and confirmation in a cell-free transcription/translation assay. A, phospho-Bim-binding proteins were precipitated from rat cortical neurons and subjected to one-dimensional PAGE followed by mass spectroscopy. The peptide R.QTIIGQPMSVTITK.D was identified by mass spectrometry as a phospho-Bim-binding protein. This peptide corresponds to the rat homologue of TRIM2. Because this was the only E3 ligase protein identified, it was considered further. B, binding of TRIM2 and Bim was confirmed by immunoprecipitation. Rat brain was incubated with GST-Bim and then subjected to TRIM2 immunoprecipitation. GST-Bim was identified by immunoblotting precipitated (IP) proteins with a GST-specific antibody. As a control the TRIM2 antibody was omitted from the reaction (−ve). C, using a cell-free expression system (Promega), TRIM2 and a control protein (p53 binding domain) DNA clones were synthesized into proteins. Expression was confirmed by immunoblotting (IB) lysate samples with a c-myc-specific antibody. D, GST-Bim was incubated in the absence or presence of MAPK with either myc-TRIM2 or myc-P53. As a control, a nonphosphorylatable mutant Bim (3ABim) was incubated with myc-TRIM2. Specific binding was determined by performing a GST pulldown and immunoblotting precipitated proteins for myc. Binding of myc-tagged TRIM2 increases when activated MAPK is added to the reaction. Incubation with a nonphosphorylatable BIM (3ABim) reduces this interaction. E, phosphorylation of Bim by MAPK was confirmed by blotting lysates with a Ser(P)69-specific antibody.
FIGURE 2.
Bim degradation in MEF cells is associated with an increase interaction between TRIM2 and Bim. MEF cells were serum-starved for 24 h and then incubated for 1 h with serum and emetine. A, lysates were immunoblotted for phospho-p42/p44 MAPK and Bim protein levels. B, immunoprecipitation of TRIM2 with either GST-Bim or GST3ABim following serum stimulation (Stim) of MEF cells. Pulldown is determined by immunoblotting precipitated proteins with a GST-specific antibody. Representative blots of two independent observations are shown. C, MEF cells were incubated with TPA for 1 h, and cell lysates were prepared. Some cells were co-incubated with 10 μ
m
U0126. Bim protein levels and phospho-MAPK levels were determined by immunoblotting. D, lysates from TPA-treated MEF cells were subjected to endogenous Bim immunoprecipitation. Precipitated proteins were immunoblotted for TRIM2 with a TRIM2-specific antibody (Novus). TRIM2 pulldown with Bim increased following incubation of MEF cells with TPA and was reduced by U0126.
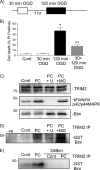
FIGURE 3.
TRIM2 binding to Bim following preconditioning ischemia is dependent on Bim phosphorylation by p42/p44 MAPK. A, schematic shows the rapid ischemic tolerance paradigm. Neuronal cultures were subjected to preconditioning ischemia (30-min OGD PC) 1 h prior to harmful ischemia (120 min OGD). B, viability was assessed in cultures subjected to 30-min, 120-min, or 30-min OGD followed by 1-h recovery and a subsequent 120-min OGD. Cell death was assessed by propidium iodide assay 24 h following the last ischemic event. Propidium iodide-positive cells are expressed as the percent of total (DAPI-stained) cells in the culture. Data shown are mean ± S.E. (error bars), n = 12. *, p < 0.001 versus control; **, p < 0.001 versus 120-min OGD (one-way ANOVA with the Bonferroni post hoc test). C, cells were subjected to 30-min OGD preconditioning ischemia and then recovered in the presence of 10 μ
m
MG132 (MG) or 10 μ
m
U0126 (U) for 1 h. TRIM2, Bim, and phospho-MAPK levels were assessed using immunoblotting. D, cells were subjected to 30-min OGD preconditioning ischemia and then recovered in the presence of 10 μ
m
MG132 or 10 μ
m
U0126 for 1 h. Lysates were incubated with GST-Bim and then subjected to TRIM2 immunoprecipitation (TRIM2 IP). Precipitated proteins were immunoblotted with a GST-specific antibody. Binding of Bim with TRIM2 increases following 30-min OGD and is reduced by U0126. E, cells were subjected to 30-min preconditioning ischemia. Lysates were prepared and incubated with GST-Bim or GST-3ABim. Following TRIM2 immunoprecipitation (TRIM2 IP), precipitated proteins were immunoblotted with a GST-specific antibody. The pulldown of GST-Bim from cell lysates by TRIM2 (TRIM2 IP) is increased following preconditioning ischemia. Nonphosphorylatable 3ABim did not pull down.
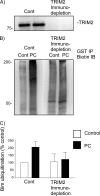
FIGURE 4.
Immunodepletion of TRIM2 from preconditioned neuronal lysates reduces Bim ubiquitination. Cell lysates from control and preconditioned cells (30-min OGD, 1-h recovery) were prepared and subjected to TRIM2 or control (CBP) immunodepletion. Antibodies raised against CREB binding protein or TRIM2 were bound to protein A/G-agarose beads and incubated with prepared lysates. A, to confirm removal of TRIM2 from the lysate, a sample (5%) was subjected to immunoblotting with a TRIM2-specific antibody. B, control and TRIM2-immunodepleted lysates were then used to measure Bim ubiquitination. Cell lysates were incubated with GST-Bim, biotinylated ubiquitin, and E1 and E2 proteins. GST-Bim was precipitated by GST pulldown, and the ubiquitinated precipitated proteins were immunoblotted with a biotin-specific antibody. Preconditioning results in Bim ubiquitination in control (CBP) immunodepleted lysates, but not TRIM2 depleted lysates. The white dotted line denotes where the image was cut and reassembled to match the above lane loading. C, quantification of three independent experiments is shown.
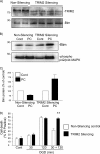
FIGURE 5.
Lentiviral-mediated delivery of anti-TRIM2 shRNAmir reveals a role of TRIM2 in rapid ischemic tolerance-induced neuroprotection. A, neuronal cells were incubated with control or anti-TRIM2 shRNAmir (multiplicity of infection of 10, 48 h). Cell lysates were prepared, and TRIM2 and Bim protein levels were determined by immunoblotting. B, cells were incubated with either nonsilencing control or TRIM2-silencing shRNAmir for 48 h. Cells were then preconditioned with 30-min OGD and recovered for 1 h. Bim protein levels and MAPK phosphorylation were determined by immunoblotting. Representative immunoblots are shown for Bim and phosphorylated p42/p44 MAPK in TRIM2 knockdown cells and control cells subjected to 30-min OGD and 1-h recovery. C, cells were incubated with TRIM2 shRNAmir or nonsilencing control for 48 h. Cells were then subjected to 30-min, 120-min or 30-min followed by 1-h recovery and a subsequent 120-min OGD. Cell death was assessed by propidium iodide assay 24 h later. Data shown are mean ± S.E. (error bars), n = 5. **, p < 0.001 versus nonsilencing treated cells (two-way ANOVA with Bonferroni post hoc test).
Similar articles
- Rapid degradation of Bim by the ubiquitin-proteasome pathway mediates short-term ischemic tolerance in cultured neurons.
Meller R, Cameron JA, Torrey DJ, Clayton CE, Ordonez AN, Henshall DC, Minami M, Schindler CK, Saugstad JA, Simon RP. Meller R, et al. J Biol Chem. 2006 Mar 17;281(11):7429-36. doi: 10.1074/jbc.M512138200. Epub 2006 Jan 23. J Biol Chem. 2006. PMID: 16431916 Free PMC article. - c-Cbl is not required for ERK1/2-dependent degradation of BimEL.
Wiggins CM, Band H, Cook SJ. Wiggins CM, et al. Cell Signal. 2007 Dec;19(12):2605-11. doi: 10.1016/j.cellsig.2007.08.008. Epub 2007 Aug 23. Cell Signal. 2007. PMID: 17884340 Free PMC article. - BIM(EL), an intrinsically disordered protein, is degraded by 20S proteasomes in the absence of poly-ubiquitylation.
Wiggins CM, Tsvetkov P, Johnson M, Joyce CL, Lamb CA, Bryant NJ, Komander D, Shaul Y, Cook SJ. Wiggins CM, et al. J Cell Sci. 2011 Mar 15;124(Pt 6):969-77. doi: 10.1242/jcs.058438. J Cell Sci. 2011. PMID: 21378313 - Expression and Role of TRIM2 in Human Diseases.
Xiao M, Li J, Liu Q, He X, Yang Z, Wang D. Xiao M, et al. Biomed Res Int. 2022 Aug 23;2022:9430509. doi: 10.1155/2022/9430509. eCollection 2022. Biomed Res Int. 2022. PMID: 36051486 Free PMC article. Review. - Posttranslational regulation of Bim by caspase-3.
Wakeyama H, Akiyama T, Kadono Y, Nakamura M, Oshima Y, Nakamura K, Tanaka S. Wakeyama H, et al. Ann N Y Acad Sci. 2007 Nov;1116:271-80. doi: 10.1196/annals.1402.001. Epub 2007 Jun 21. Ann N Y Acad Sci. 2007. PMID: 17584989 Review.
Cited by
- Regulation of apoptosis by ubiquitination in liver cancer.
Li Y, Zhu J, Yu Z, Zhai F, Li H, Jin X. Li Y, et al. Am J Cancer Res. 2023 Oct 15;13(10):4832-4871. eCollection 2023. Am J Cancer Res. 2023. PMID: 37970337 Free PMC article. Review. - Expression of the Phosphatase Ppef2 Controls Survival and Function of CD8+ Dendritic Cells.
Zwick M, Ulas T, Cho YL, Ried C, Grosse L, Simon C, Bernhard C, Busch DH, Schultze JL, Buchholz VR, Stutte S, Brocker T. Zwick M, et al. Front Immunol. 2019 Feb 12;10:222. doi: 10.3389/fimmu.2019.00222. eCollection 2019. Front Immunol. 2019. PMID: 30809231 Free PMC article. - Gene expression-phenotype associations in adults with eosinophilic esophagitis.
Dellon ES, Selitsky SR, Genta RM, Lash RH, Parker JS. Dellon ES, et al. Dig Liver Dis. 2018 Aug;50(8):804-811. doi: 10.1016/j.dld.2018.03.021. Epub 2018 Mar 27. Dig Liver Dis. 2018. PMID: 29628359 Free PMC article. - The ubiquitin/proteasome system-dependent control of mitochondrial steps in apoptosis.
Neutzner A, Li S, Xu S, Karbowski M. Neutzner A, et al. Semin Cell Dev Biol. 2012 Jul;23(5):499-508. doi: 10.1016/j.semcdb.2012.03.019. Epub 2012 Apr 11. Semin Cell Dev Biol. 2012. PMID: 22516642 Free PMC article. Review. - TRIM2, a novel member of the antiviral family, limits New World arenavirus entry.
Sarute N, Ibrahim N, Medegan Fagla B, Lavanya M, Cuevas C, Stavrou S, Otkiran-Clare G, Tyynismaa H, Henao-Mejia J, Ross SR. Sarute N, et al. PLoS Biol. 2019 Feb 6;17(2):e3000137. doi: 10.1371/journal.pbio.3000137. eCollection 2019 Feb. PLoS Biol. 2019. PMID: 30726215 Free PMC article.
References
- Nakamura M., Nakakimura K., Matsumoto M., Sakabe T. (2002) J. Cereb. Blood Flow Metab. 22, 161–170 - PubMed
- Reshef A., Sperling O., Zoref-Shani E. (2000) Adv. Exp. Med. Biol. 486, 217–221 - PubMed
- Reshef A., Sperling O., Zoref-Shani E. (2000) Neuroreport 11, 463–465 - PubMed
- Pérez-Pinzón M. A., Born J. G. (1999) Neuroscience 89, 453–459 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21 NS050669-01/NS/NINDS NIH HHS/United States
- R01 NS059588-01A2/NS/NINDS NIH HHS/United States
- NS59588/NS/NINDS NIH HHS/United States
- R21 NS050669/NS/NINDS NIH HHS/United States
- G12-RR03034/RR/NCRR NIH HHS/United States
- R01 NS039016/NS/NINDS NIH HHS/United States
- NS24728/NS/NINDS NIH HHS/United States
- NS39016/NS/NINDS NIH HHS/United States
- G12 RR003034/RR/NCRR NIH HHS/United States
- R01 NS024728/NS/NINDS NIH HHS/United States
- S21 MD000101/MD/NIMHD NIH HHS/United States
- R01 NS059588/NS/NINDS NIH HHS/United States
- U54 NS060659/NS/NINDS NIH HHS/United States
- NS50669/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials