Promyelocytic leukemia zinc finger turns on the effector T cell program without requirement for agonist TCR signaling - PubMed (original) (raw)
Promyelocytic leukemia zinc finger turns on the effector T cell program without requirement for agonist TCR signaling
Adam K Savage et al. J Immunol. 2011.
Abstract
Thymocytes expressing the NKT cell semi-invariant αβ TCR are thought to undergo agonist interactions with CD1d ligands prior to expressing promyelocytic leukemia zinc finger (PLZF), a broad complex, tramtrack, bric-a-brac, poxvirus, and zinc finger transcription factor that directs acquisition of the effector program of these innate-like T cells. Whether PLZF can mediate this effector conversion independently of agonist signaling has not been investigated. We demonstrated that transgenic (Tg) expression of PLZF under the CD4 promoter induced the innate effector program in two different MHC class II-restricted TCR-Tg Rag1(-/-) models examined. In CD4 thymocytes expressing a fixed Tg TCR β-chain, the associated TCRα sequences in wild-type and PLZF-Tg mice overlapped extensively, further demonstrating that PLZF could induce the effector program in most CD4 T cells that would normally be selected as naive cells. In contrast, PLZF altered the negative selection of thymocytes expressing TCR β-chains reactive against several retroviral superantigens. Thus, PLZF is remarkable in that it is a transcription factor capable of inducing an effector program in the absence of T cell agonist interactions or cell division. Its expression may also enhance the survival of agonist-signaled thymocytes.
Figures
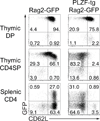
Figure 1. PLZF-transgenic CD4 SP thymocytes permanently downregulate CD62L prior to emigration
PLZF-transgenic mice were bred to Rag2p-GFP-transgenic mice and the progeny was analyzed at 4–7 weeks of age. The plots show expression of GFP and CD62L among CD4+CD8+ (DP) and CD4+CD8− (CD4SP) thymocytes or TCRβ+CD4+ (CD4) splenocytes. Data are representative of three experiments.
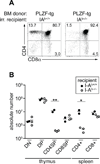
Figure 2. PLZF-transgenic CD4 cells are selected by epithelial MHC II ligands
PLZF-transgenic bone marrow was transferred into lethally irradiated I-Ab+/+ or I-Ab−/− recipients. (A) representative data from the thymus at 7 weeks post-transfer. (B) summary of T cell subset numbers in the thymus and spleen of individual chimeras. DN: CD4−CD8−, DP: CD4+CD8+. A similar requirement for epithelial MHC ligand was observed with another set of bone marrow chimeras where PLZF-transgenic bone marrow was injected into MHC-I and II deficient mice (β2M−/−IAb−/−). *p<0.05, **p<0.01.
Figure 3. PLZF expression converts conventional TCR-transgenic CD4 cells into effectors
Marilyn (I-Ab-HY specific) and OTII (I-Ab-OVA specific) TCR-transgenic mice in a Rag1−/− background were analyzed at 4–8 weeks for expression of CD44 and CD62L on CD4+Vβ6+ (Marilyn) or CD4+Vβ5+ (OTII) thymocytes and splenocytes. 3/3 Marilyn and 4/4 OTII mice examined showed similar effector conversion.
Figure 4. PLZF expression does not alter the TCR repertoire
Vβ7-transgenic CD4+CD8− (CD4SP) or CD4−CD8+ (CD8SP) thymocytes on a WT or PLZF-transgenic background were FACS sorted to isolate Vα2 expressing cells and determine individual Vα2-Cα junctional sequences. Between 216 and 277 sequences were determined for each population and stacked in a bar graph where horizontal slices represent different aminoacid sequences with the height corresponding to the number of instances of that sequence; identical sequences found in different populations are connected and in the same color. (D) CD4SP thymocytes of two WT mice (average 38.6% shared unique sequences); (E), CD4SP and CD8SP thymocytes of a single WT mouse (average 6.5% shared unique sequences); (F), CD4SP thymocytes of a PLZF-transgenic and a WT littermate (average 33.3% shared unique sequences). Averages are calculated from two separate experiments including a pair of WT and transgenic littermates.
Figure 5. Negative selection in PLZF-transgenic mice
(A) Influence of PLZF expression in the thymus of Marilyn (I-Ab-HY specific) TCR transgenic male and female mice examined at 4.5 weeks of age. Numbers above the dot plots indicate thymic cellularity. Data is representative of two experiments. (B) Influence of PLZF on endogenous retroviral superantigen-induced deletion of Vβ-expressing thymocytes and splenocytes. 4–8 week-old C57BL/6 × BALB/c F1 hybrid mice were analyzed for the expression of Vβ families among CD3hi thymocytes and splenocytes. F1 mice delete Vβ5, Vβ11 and Vβ12, but not Vβ14, due to endogenous viral superantigens in the BALB/c background. (C) Summary plots showing the percentage of Vβ-expressing CD3+CD4+CD8− thymocytes in individual mice as depicted in B. ****p<0.0001.
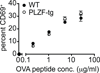
Figure 6. PLZF does not alter TCR signaling in DP thymocytes
CD69-depleted thymocytes from either PLZF-transgenic OTII-transgenic Rag1−/− or OTII-transgenic Rag1+/− were stimulated with T-cell depleted splenocytes and cultured for 20 hours with varying concentrations of OTII peptide (0, 0.2, 1, 5, 25 mg/ml). The chart shows the mean percentage of CD69+ cells (from duplicate wells) among CD4+CD8+ cells. The standard error of the mean is indicated by the error bars. Data are representative of two independent experiments conducted using littermates at 4 and 8 weeks of age.
Similar articles
- Innate PLZF+CD4+ αβ T cells develop and expand in the absence of Itk.
Prince AL, Watkin LB, Yin CC, Selin LK, Kang J, Schwartzberg PL, Berg LJ. Prince AL, et al. J Immunol. 2014 Jul 15;193(2):673-87. doi: 10.4049/jimmunol.1302058. Epub 2014 Jun 13. J Immunol. 2014. PMID: 24928994 Free PMC article. - A transgenic TCR directs the development of IL-4+ and PLZF+ innate CD4 T cells.
Zhu L, Qiao Y, Choi ES, Das J, Sant'angelo DB, Chang CH. Zhu L, et al. J Immunol. 2013 Jul 15;191(2):737-44. doi: 10.4049/jimmunol.1300862. Epub 2013 Jun 17. J Immunol. 2013. PMID: 23776174 Free PMC article. - PLZF induces the spontaneous acquisition of memory/effector functions in T cells independently of NKT cell-related signals.
Kovalovsky D, Alonzo ES, Uche OU, Eidson M, Nichols KE, Sant'Angelo DB. Kovalovsky D, et al. J Immunol. 2010 Jun 15;184(12):6746-55. doi: 10.4049/jimmunol.1000776. Epub 2010 May 21. J Immunol. 2010. PMID: 20495068 - Promyelocytic leukemia zinc finger controls type 2 immune responses in the lungs by regulating lineage commitment and the function of innate and adaptive immune cells.
Sha J, Zhang M, Feng J, Shi T, Li N, Jie Z. Sha J, et al. Int Immunopharmacol. 2024 Mar 30;130:111670. doi: 10.1016/j.intimp.2024.111670. Epub 2024 Feb 18. Int Immunopharmacol. 2024. PMID: 38373386 Review. - Development of PLZF-expressing innate T cells.
Alonzo ES, Sant'Angelo DB. Alonzo ES, et al. Curr Opin Immunol. 2011 Apr;23(2):220-7. doi: 10.1016/j.coi.2010.12.016. Epub 2011 Jan 21. Curr Opin Immunol. 2011. PMID: 21257299 Free PMC article. Review.
Cited by
- ZBTB Transcription Factors: Key Regulators of the Development, Differentiation and Effector Function of T Cells.
Cheng ZY, He TT, Gao XM, Zhao Y, Wang J. Cheng ZY, et al. Front Immunol. 2021 Jul 19;12:713294. doi: 10.3389/fimmu.2021.713294. eCollection 2021. Front Immunol. 2021. PMID: 34349770 Free PMC article. Review. - Forging T-Lymphocyte Identity: Intersecting Networks of Transcriptional Control.
Rothenberg EV, Ungerbäck J, Champhekar A. Rothenberg EV, et al. Adv Immunol. 2016;129:109-74. doi: 10.1016/bs.ai.2015.09.002. Epub 2015 Oct 26. Adv Immunol. 2016. PMID: 26791859 Free PMC article. Review. - Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions.
Brennan PJ, Brigl M, Brenner MB. Brennan PJ, et al. Nat Rev Immunol. 2013 Feb;13(2):101-17. doi: 10.1038/nri3369. Epub 2013 Jan 21. Nat Rev Immunol. 2013. PMID: 23334244 Review. - Multiple layers of transcriptional regulation by PLZF in NKT-cell development.
Mao AP, Constantinides MG, Mathew R, Zuo Z, Chen X, Weirauch MT, Bendelac A. Mao AP, et al. Proc Natl Acad Sci U S A. 2016 Jul 5;113(27):7602-7. doi: 10.1073/pnas.1601504113. Epub 2016 Jun 20. Proc Natl Acad Sci U S A. 2016. PMID: 27325774 Free PMC article. - A committed precursor to innate lymphoid cells.
Constantinides MG, McDonald BD, Verhoef PA, Bendelac A. Constantinides MG, et al. Nature. 2014 Apr 17;508(7496):397-401. doi: 10.1038/nature13047. Epub 2014 Feb 9. Nature. 2014. PMID: 24509713 Free PMC article.
References
- Bendelac A, Bonneville M, Kearney JF. Autoreactivity by design: innate B and T lymphocytes. Nat Rev Immunol. 2001;1:177–186. - PubMed
- Matsuda JL, Gapin L. Developmental program of mouse Valpha14i NKT cells. Curr Opin Immunol. 2005;17:122–130. - PubMed
- Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. - PubMed
- Godfrey DI, Berzins SP. Control points in NKT-cell development. Nat Rev Immunol. 2007;7:505–518. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HHMI/Howard Hughes Medical Institute/United States
- R01 AI038339/AI/NIAID NIH HHS/United States
- R37 AI038339/AI/NIAID NIH HHS/United States
- AI038339/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous