Targeted treatments for fragile X syndrome - PubMed (original) (raw)
Targeted treatments for fragile X syndrome
Elizabeth Berry-Kravis et al. J Neurodev Disord. 2011 Sep.
Abstract
Fragile X syndrome (FXS) is the most common identifiable genetic cause of intellectual disability and autistic spectrum disorders (ASD), with up to 50% of males and some females with FXS meeting criteria for ASD. Autistic features are present in a very high percent of individuals with FXS, even those who do not meet full criteria for ASD. Recent major advances have been made in the understanding of the neurobiology and functions of FMRP, the FMR1 (fragile X mental retardation 1) gene product, which is absent or reduced in FXS, largely based on work in the fmr1 knockout mouse model. FXS has emerged as a disorder of synaptic plasticity associated with abnormalities of long-term depression and long-term potentiation and immature dendritic spine architecture, related to the dysregulation of dendritic translation typically activated by group I mGluR and other receptors. This work has led to efforts to develop treatments for FXS with neuroactive molecules targeted to the dysregulated translational pathway. These agents have been shown to rescue molecular, spine, and behavioral phenotypes in the FXS mouse model at multiple stages of development. Clinical trials are underway to translate findings in animal models of FXS to humans, raising complex issues about trial design and outcome measures to assess cognitive change that might be associated with treatment. Genes known to be causes of ASD interact with the translational pathway defective in FXS, and it has been hypothesized that there will be substantial overlap in molecular pathways and mechanisms of synaptic dysfunction between FXS and ASD. Therefore, targeted treatments developed for FXS may also target subgroups of ASD, and clinical trials in FXS may serve as a model for the development of clinical trial strategies for ASD and other cognitive disorders.
Figures
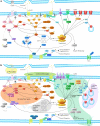
Fig. 1
Synaptic translation and signaling pathways modulated by FMRP (a) and dysregulation of these pathways in the absence or significant reduction of FMRP (b). Shaded areas in (b) indicate groups of targets for different strategies aimed at treatment of FXS by correcting dysregulated neuronal pathways. Shaded areas are numbered according to type of treatment strategy and correspond to numbering system for treatment strategies in the text as follows: (1) reduction of activity in pathways that transduce signals from group 1 mGluRs or other Gq-linked receptors to the dendritic translational machinery via (1a) extracellular pathway (receptor) blockers and/or (1b) intracellular pathway blockers; (2) reduction of activity of individual proteins regulated by FMRP; (3) increasing surface AMPA receptors and/or activity; (4) modification of activity of other receptors/proteins that regulate synaptic activity; and (5) blocking translation of mRNAs regulated by FMRP using antisense technology
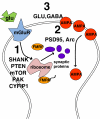
Fig. 2
Classes of potential overlap in synaptic mechanisms between ASD genes/proteins and pathways involved in FXS: (1) proteins involved in other forms of ASD may be in the signaling cascade for activation of FMRP-regulated translation; (2) FMRP may directly regulate proteins involved in different forms of ASD; and (3) convergence of glutamate and GABA pathway defects in FXS and ASD due to dysregulation of proteins generally important in maintaining inhibitory/excitatory balance and balance of activity in brain glutamate and GABA systems
Similar articles
- Mechanism-based treatments in neurodevelopmental disorders: fragile X syndrome.
Berry-Kravis E. Berry-Kravis E. Pediatr Neurol. 2014 Apr;50(4):297-302. doi: 10.1016/j.pediatrneurol.2013.12.001. Epub 2013 Dec 4. Pediatr Neurol. 2014. PMID: 24518745 Review. - Deletion of Fmr1 in parvalbumin-expressing neurons results in dysregulated translation and selective behavioral deficits associated with fragile X syndrome.
Kalinowska M, van der Lei MB, Kitiashvili M, Mamcarz M, Oliveira MM, Longo F, Klann E. Kalinowska M, et al. Mol Autism. 2022 Jun 29;13(1):29. doi: 10.1186/s13229-022-00509-2. Mol Autism. 2022. PMID: 35768828 Free PMC article. - Fragile X-like behaviors and abnormal cortical dendritic spines in cytoplasmic FMR1-interacting protein 2-mutant mice.
Han K, Chen H, Gennarino VA, Richman R, Lu HC, Zoghbi HY. Han K, et al. Hum Mol Genet. 2015 Apr 1;24(7):1813-23. doi: 10.1093/hmg/ddu595. Epub 2014 Nov 28. Hum Mol Genet. 2015. PMID: 25432536 Free PMC article. - FMR1 and Autism, an Intriguing Connection Revisited.
Fyke W, Velinov M. Fyke W, et al. Genes (Basel). 2021 Aug 6;12(8):1218. doi: 10.3390/genes12081218. Genes (Basel). 2021. PMID: 34440392 Free PMC article. Review. - Proteome profiling of the prefrontal cortex of Fmr1 knockout mouse reveals enhancement of complement and coagulation cascades.
Gao MM, Shi H, Yan HJ, Long YS. Gao MM, et al. J Proteomics. 2023 Mar 15;274:104822. doi: 10.1016/j.jprot.2023.104822. Epub 2023 Jan 13. J Proteomics. 2023. PMID: 36646274
Cited by
- Early identification of autism in fragile X syndrome: a review.
McCary LM, Roberts JE. McCary LM, et al. J Intellect Disabil Res. 2013 Sep;57(9):803-14. doi: 10.1111/j.1365-2788.2012.01609.x. Epub 2012 Sep 14. J Intellect Disabil Res. 2013. PMID: 22974167 Free PMC article. Review. - Rescue of dendritic spine phenotype in Fmr1 KO mice with the mGluR5 antagonist AFQ056/Mavoglurant.
Pop AS, Levenga J, de Esch CE, Buijsen RA, Nieuwenhuizen IM, Li T, Isaacs A, Gasparini F, Oostra BA, Willemsen R. Pop AS, et al. Psychopharmacology (Berl). 2014 Mar;231(6):1227-35. doi: 10.1007/s00213-012-2947-y. Epub 2012 Dec 21. Psychopharmacology (Berl). 2014. PMID: 23254376 - An experimentally validated approach to automated biological evidence generation in drug discovery using knowledge graphs.
Sudhahar S, Ozer B, Chang J, Chadwick W, O'Donovan D, Campbell A, Tulip E, Thompson N, Roberts I. Sudhahar S, et al. Nat Commun. 2024 Jul 8;15(1):5703. doi: 10.1038/s41467-024-50024-6. Nat Commun. 2024. PMID: 38977662 Free PMC article. - The role of ionotropic glutamate receptors in childhood neurodevelopmental disorders: autism spectrum disorders and fragile x syndrome.
Uzunova G, Hollander E, Shepherd J. Uzunova G, et al. Curr Neuropharmacol. 2014 Jan;12(1):71-98. doi: 10.2174/1570159X113116660046. Curr Neuropharmacol. 2014. PMID: 24533017 Free PMC article. - Psychophysiological responses to emotional stimuli in children and adolescents with autism and fragile X syndrome.
Cohen S, Masyn K, Mastergeorge A, Hessl D. Cohen S, et al. J Clin Child Adolesc Psychol. 2015;44(2):250-63. doi: 10.1080/15374416.2013.843462. Epub 2013 Oct 24. J Clin Child Adolesc Psychol. 2015. PMID: 24156344 Free PMC article.
References
- Adusei DC, Pacey LK, Chen D, Hampson DR. Early developmental alterations in GABAergic protein expression in fragile X knockout mice. Neuropharmacology. 2010;59:167–171. - PubMed
- Bagni C, Greenough WT. From mRNP trafficking to spine dysmorphogenesis: the roots of fragile X syndrome. Nat Rev Neurosci. 2005;6:376–387. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources